Team advances fuel cell car technology
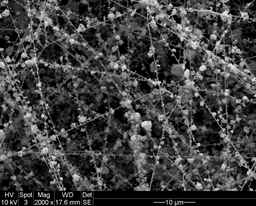
Dr. Yossef Elabd, professor in the Artie McFerrin Department of Chemical Engineering at Texas A&M University, has developed two fuel cell vehicle platforms for both present day enhancements and future innovation.
Electrochemical energy and polymer research unite in new ways to deliver best-case power scenarios behind the wheel, specifically under the hood. Environmentalists and road aficionados alike have applauded the production of fuel cell vehicles, but to sustain the appeal and increase the market, production costs need to fall.
A fuel cell is a device that produces electric energy as the direct result of a chemical reaction. The fuel cell car is powered by the constant chemical interaction of hydrogen fuel from the tank and oxygen that is pulled from the air. Using positive (anode) and negative (cathode) electrodes, protons are moved by an electrolyte and ultimately converted into energy. The source and form of the electrodes and electrolytes can vary and this is the basis of Elabd's findings.
The fuel cell car manufactured today uses a proton exchange membrane (PEM) electrolyte for its platinum-based electrodes, the same design that was used in previous Gemini space missions.
Elabd has discovered that the amount of platinum needed for electrode manufacturing can be significantly reduced, and thus the production costs dramatically minimized. His patent-pending research establishes that a fuel cell can perform optimally with only 16 percent of its previous platinum requirements. New polymer nanofiber-platinum nanoparticle electrodes are reliable and enduring, he said.
Another course of action, Elabd envisions, is the creation of a fuel cell that does not require platinum. This would be "the holy grail of portable power."
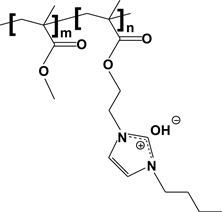
The alkaline exchange membrane (AEM) fuel cell model uses a polymer material as its electrolyte. Past developers encountered roadblocks (chemical degradation) in the introduction of a polymer to an AEM fuel cell, yet Elabd's research group has achieved patented breakthrough in developing a polymer electrolyte that is chemically stable to the hydroxide ions, highly conductive in moving the ions across the fuel cell and very robust.
"The same material if modified a little bit can be used in batteries as a replacement to current lithium-ion charged batteries, making it a solid state with the benefit of longer stability and much increased safety," Elabd said.
Whether the approach is to reduce platinum requirements or to implement a polymer electrolyte, both solutions appear to have passed the proof of concept phase.
"I just want to drive my car with water vapor coming out the back of it," Elabd said.
Environmentalists everywhere agree.
Provided by Texas A&M University




















