New material, technique efficiently produce hydrogen, syngas fuel feedstock

A team of chemical engineering researchers has developed a technique that uses a new catalyst to convert methane and water into hydrogen and a fuel feedstock called syngas with the assistance of solar power. The catalytic material is more than three times more efficient at converting water into hydrogen gas than previous thermal water-splitting methods.
"We're excited about the new material and process because it converts water, inexpensive natural gas and clean, renewable solar energy into valuable syngas and hydrogen fuels," says Feng He, a Ph.D. student in the lab of Prof. Fanxing Li at NC State and lead author of two articles describing the material and process.
Hydrogen may be an important source of clean energy, and the cleanest way to produce hydrogen gas is to split water into hydrogen and oxygen – but researchers have struggled to develop a cost-effective water-splitting technique. Syngas is a mixture of carbon monoxide and hydrogen that is used as a feedstock for commercial processes that produce synthetic diesel fuels, olefins, and methanol.
The technique hinges on a new catalytic material that is a composite of iron oxide and lanthanum strontium iron oxide, also known as LSF.
Researchers have long known that iron oxide can be used as a catalyst for thermal water splitting, but it is not very efficient. The addition of LSF significantly improves iron oxide's activity, making it far more efficient. Using the new composite, the researchers were able to convert 77 percent of the water they used (in the form of steam) into hydrogen. The previous best conversion mark for thermal water-splitting was around 20 percent.
"We're optimistic that commercial utilization of this technique could promote the efficient usage of solar energy and domestic natural gas, produce relatively low carbon dioxide emissions while making liquid transportation fuel, and generate low cost, high purity hydrogen," He says.
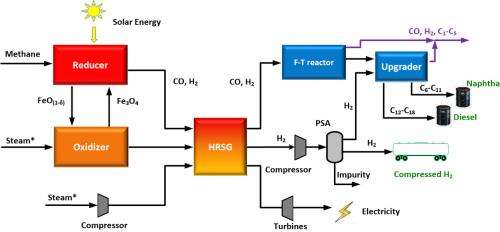
Broadly speaking, here's how the new technique works.
Methane is injected into a reactor that is heated with solar energy. That chamber contains the catalytic composite, which reacts with the methane to produce syngas and carbon dioxide. This process "reduces" the composite particles, stripping them of oxygen. The syngas is removed from the system and the reduced composite particles are diverted into a second reactor.
High-temperature steam is then pumped into the second reactor, where it reacts with the reduced composite particles to produce hydrogen gas that is at least 97 percent pure (which is good). This process also reoxygenates the composite particles, which can then be re-used with the methane, starting the cycle all over again.
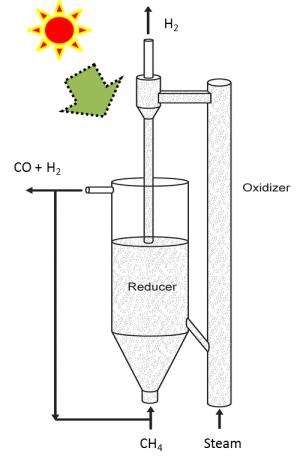
Initially, the steam has to be produced with an external energy source, but once the cycle is initiated the chemical reactions produce enough heat to convert water into steam without an external heat source.
"We've created the catalytic particles and conducted every step of this process, but only in separate batches," He says. "We're now in the process of building a circulating bed reactor to operate this entire cycle in a continuous mode in real world conditions.
"Next steps include fine-tuning the catalytic compound to make it better and cheaper, improving the overall process, and developing better reactors."
More information: "Perovskite promoted iron oxide for hybrid water-splitting and syngas generation with exceptional conversion." Energy Environ. Sci., 2015, Advance Article DOI: 10.1039/C4EE03431G
"A hybrid solar-redox scheme for liquid fuel and hydrogen coproduction." Energy Environ. Sci.,, 2014,7, 2033-2042 DOI: 10.1039/C4EE00038B
Provided by North Carolina State University




















