Study gets to the roots of plants' natural sunscreen

(Phys.org) —Plants bask in the sun and need its light to live, but they also coat themselves in a natural sunscreen like a sunbather on the beach, protecting themselves from damaging rays.
A new study examined the properties and mechanics of the molecule plants use to absorb harmful ultraviolet-B radiation, and its SPF rating would be off the charts.
"This molecule is a fantastic sunscreen and can absorb a remarkably broad spectrum of UV-B light – the entire spectrum," said Timothy Zwier, Purdue University's M.G. Mellon Distinguished Professor of Chemistry, who led the study. "It also is incredibly good at soaking up those rays, with each molecule capturing an impressive amount of UV-B light."
The research team studied molecules from a group called sinapate esters that have been shown to be involved in protecting plants from UV-B light that can damage DNA and plant tissues.
"I was intrigued by the presentation of a Purdue colleague, Clint Chapple, who discovered that these sinapate esters act as a plant sunscreen," Zwier said. "He showed how plants that had been modified to be unable to produce these molecules suffered crippling damage that led to poor growth and withering. I found the notion of plant sunscreen fascinating. My research group wanted to explore why these particular molecules were being used by the plants, and we thought we had the tools to explore the molecules and uncover what makes them special in a new way."
It was known that plants produce these molecules and send them to the surface of leaves, but exactly what wavelengths of light the molecules absorbed in isolation, and how they do it had not been determined, he said.
A paper detailing the results of the U.S. Department of Energy-funded study was published in the Journal of the American Chemical Society and is currently available online.
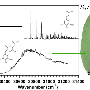
The research team used laser-based methods to record the spectrum of light absorbed by the molecules. The team was especially interested in the molecule sinapoyl malate, which was thought to be the major source of protection from the sun. Sinapoyl malate is complicated and has several different parts to it, so the team also studied similar sinapate ester molecules that retained different portions of the target molecule, Zwier said.
"This deconstructive approach is similar to an artist drawing a simple tree: First you draw the trunk, then you add a branch and then another branch and so on until you have the complete picture," he said. "This also helps us pinpoint which parts of the molecule are most essential to its unique properties."
The researchers first used a laser to bring the molecule out of its solid state and into the gas phase. They then cooled the molecule to temperatures near absolute zero to isolate it and make its spectrum as clear and easy to interpret as possible, he said. The team used three different lasers to analyze the different shapes the molecules would take on as they absorbed different wavelengths of UV-B light. They also analyzed the structures of the molecules using infrared light.
The researchers found sinapoyl malate's absorption efficiency - a measure of how many molecules it takes to absorb a given amount of light - was one of the highest that can be achieved, Zwier said. The absorption efficiency is quantified as a molecule's oscillator strength, and sinapoyl malate achieved a strength of 0.65, where 1.0 is the highest value possible.
"This is about the biggest absorption efficiency you can find in a molecule," he said. "So it does an excellent job capturing the UV-B light while letting other wavelengths of light necessary to plant life slip right through. Our findings confirm sinapoyl malate has this key ingredient for functioning as an efficient plant sunscreen."
What most surprised Zwier and his team was that, despite their extraordinary efforts to isolate sinapoyl malate by stripping away surrounding molecules and removing its thermal energy, it retained an inherently broadened UV spectrum.
"This molecule absorbs all wavelengths of UV-B radiation with no gaps in coverage," he said. "Other molecules that are very similar to sinapoyl malate have gaps in their spectra that let some of the UV-B light slip through. This broad spectrum is exactly what is needed for a good sunscreen, that all wavelengths of harmful radiation are absorbed with high efficiency."
Chapple, a distinguished professor and head of Purdue's Department of Biochemistry, also found the broad coverage of sinapoyl malate significant.
"Natural selection has identified a compound that has UV absorbance characteristics that make it particularly well-suited to function as a suncscreen," Chapple said.
Zwier and his team proposed that some of this complete wavelength coverage is due to the presence of a second excited state that works in concert with the first one to fill in any gaps in wavelength coverage that might otherwise exist.
"As often occurs in science, answering some questions raises others," Zwier said. "While it is evident that sinapoyl malate is absorbing this light and energy, the bigger question remains of what happens to it next."
The best-case scenario is that the energy is funneled back down into the lower energy state of the molecule, eventually turning the energy from the light into heat. However, a molecule in an excited state and loaded with energy is also poised for involvement in harmful reactions, he said.
The team plans to further study the molecule using complementary techniques to better understand the processes involved.
This fundamental research is critical for other agricultural research, Chapple said.
"As our environment changes and we manipulate plants through breeding to optimize productivity, it is critical that we have a full understanding of how plants operate at every level, including UV protection," he said. "These results serve as a fascinating cautionary tale. Without this sort of very fundamental research, we might erroneously manipulate plant metabolic pathways. For example, we might decrease the content of one compound while increasing the content of another – thinking them to be interchangeable – without realizing that the one that had been selected by nature had unique characteristiscs."
More information: "Plant Sunscreens in the UV-B: Ultraviolet Spectroscopy of Jet-Cooled Sinapoyl Malate, Sinapic Acid, and Sinapate Ester Derivatives" J. Am. Chem. Soc., 2014, 136 (42), pp 14780–14795. DOI: 10.1021/ja5059026
Journal information: Journal of the American Chemical Society
Provided by Purdue University