Rethinking basic science of graphene synthesis shows route to industrial-scale production
A new route to making graphene has been discovered that could make the 21st century's wonder material easier to ramp up to industrial scale. Graphene—a tightly bound single layer of carbon atoms with super strength and the ability to conduct heat and electricity better than any other known material—has potential industrial uses that include flexible electronic displays, high-speed computing, stronger wind-turbine blades, and more-efficient solar cells, to name just a few under development.
In the decade since Nobel laureates Konstantin Novoselov and Andre Geim proved the remarkable electronic and mechanical properties of graphene, researchers have been hard at work to develop methods of producing pristine samples of the material on a scale with industrial potential. Now, a team of Penn State scientists has discovered a route to making single-layer graphene that has been overlooked for more than 150 years.
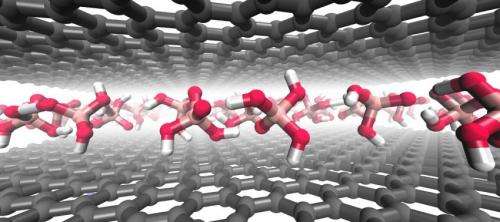
"There are lots of layered materials similar to graphene with interesting properties, but until now we didn't know how to chemically pull the solids apart to make single sheets without damaging the layers," said Thomas E. Mallouk, Evan Pugh Professor of Chemistry, Physics, and Biochemistry and Molecular Biology at Penn State. In a paper first published online on Sept. 9 in the journal Nature Chemistry, Mallouk and colleagues at Penn State and the Research Center for Exotic Nanocarbons at Shinshu University, Japan, describe a method called intercalation, in which guest molecules or ions are inserted between the carbon layers of graphite to pull the single sheets apart.
The intercalation of graphite was achieved in 1841, but always with a strong oxidizing or reducing agent that damaged the desirable properties of the material. One of the most widely used methods to intercalate graphite by oxidation was developed in 1999 by Nina Kovtyukhova, a research associate in Mallouk's lab.
While studying other layered materials, Mallouk asked Kovtyukhova to use her method, which requires a strong oxidizing agent and a mixture of acids, to open up single layers of solid boron nitride, a compound with a structure similar to graphite. To their surprise, she was able to get all of the layers to open up. In subsequent control experiments, Kovtyukhova tried leaving out various agents and found that the oxidizing agent wasn't necessary for the reaction to take place.
Mallouk asked her to try a similar experiment without the oxidizing agent on graphite, but aware of the extensive literature saying that the oxidizing agent was required, Kovtyukhova balked.
"I kept asking her to try it and she kept saying no," Mallouk said. "Finally, we made a bet, and to make it interesting I gave her odds. If the reaction didn't work I would owe her $100, and if it did she would owe me $10. I have the ten dollar bill on my wall with a nice Post-it note from Nina complimenting my chemical intuition."
Mallouk believes the results of this new understanding of intercalation in boron nitride and graphene could apply to many other layered materials of interest to researchers in the Penn State Center for Two-Dimensional and Layered Materials who are investigating what are referred to as "Materials Beyond Graphene." The next step for Mallouk and colleagues will be to figure out how to speed the reaction up in order to scale up production.
More information: Nature Chemistry "Non-oxidative intercalation and exfoliation of graphite by Brønsted acids" by Nina I. Kovtyukhova et al. DOI: 10.1038/nchem.2054
Journal information: Nature Chemistry
Provided by Pennsylvania State University