A hybrid-motor helps cells push their way through tissues
Research has uncovered how two cellular motors, previously thought to compete with each other, can actually work together to help cells squeezing through a crowded mass of cells.
The study published in PNAS [1] provides fresh understanding of how cells can combine accurate steering with a brute force mechanism in order to push through our body, essential when cells of our immune defence need to reach sites of inflammation, but detrimental during tumour metastasis or parasitic infection. The work was conducted by Dr Till Bretschneider and Dr Richard University of Warwick's Systems Biology Centre and a team at the Medical Research Laboratory of Molecular Biology in Cambridge.
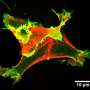
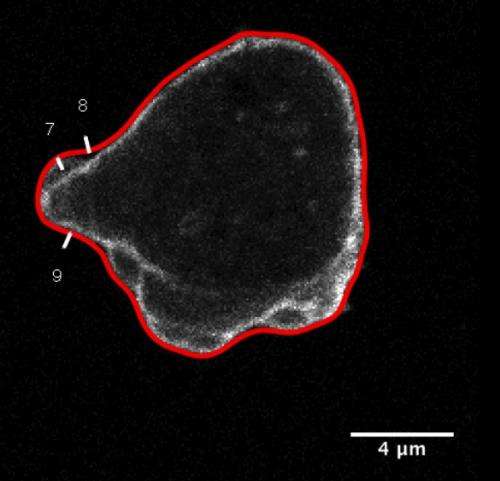
One of the cellular motors causes the cell membrane, the flexible envelope encasing all cells, to bulge out by forming so-called pseudopods. "In this instance, cell locomotion is driven by the localised growth of a dynamic protein scaffold pushing against the cell membrane from the inside," says Dr Tyson, continuing, "Cells employ complex regulation, linked to environmental sensing, to make pseudopods highly accurate steering devices, which are of limited power though."
The second motor entails faster, pressure-driven protrusions in form of cellular blebs. These provide higher force, working like a battering ram to open up gaps for cells to squeeze in-between other tightly connected cells. "Like a muscle, a cell is able to contract itself, increasing its internal pressure and causing the cell membrane to locally tear away from the underlying cytoskeletal scaffold. Pressure then blows it outwards to force aside other cells or to create footholds for traction as a rock climber would," says Dr Tyson. In contrast to pseudopods, blebs appeared to be under less precise control as to where they form on a cells' surface, making the impression of a loose cannon.
Recently Evgeny Zatulovskiy and Rob Kay from Cambridge have shown that Dictyostelium cells, a popular model organism for studying cell locomotion, can employ both motors simultaneously [2], raising the question of how they might interact.
To address this question Richard Tyson and Till Bretschneider from Warwick University developed new computer algorithms capable of tracking large numbers of both blebs and pseudopods in microscopy movies of Dictyostelium cells. In the current study, How blebs and pseudopods cooperate during chemotaxis, they demonstrate that cell shape influences how these motors interact. When slow pseudopods extend they deform the cell membrane creating an inward-curved regions at their base.
A biophysical model shows that the cell membrane, which is under tension, experiences an outward directed force in these regions, facilitating the tearing away of membrane, which precedes the formation of a bleb. "Thus, membrane geometry turns out to be an important, previously overlooked factor coupling both types of protrusions and helping to indirectly orient blebs", says Dr Bretschneider. This mechanism is similar to blistering of an overly thick coat of drying paint where moisture is trapped underneath and expands when the temperature increases.
Like the cell membrane, drying paint is also under tension, which is more easily released on inward curved surfaces, where it preferably causes paint to blister or flake off. An example of an inward curved surface where this applies would be a ceiling coving, as opposed to a plane wall. Cellular pseudopods can actively create inward curved surfaces and consequently direct where blebs form.
"The significance of this work is two-fold," Dr Bretschneider explains, "Firstly, the underlying mechanism is a generic physical one, similar to the example of drying paint. It has been confirmed to exist in different cell types and is independent of a cell's sensory system. Secondly, until now the common picture was that the requirements for forming one type of protrusion or the other are mutual exclusive. The current study shows that both motors can actually work together to make cell motility more effective by providing accurate steering to a high power motor."
More information: [1] Tyson RA, Zatulovskiy E, Kay RR, Bretschneider T. How blebs and pseudopods cooperate during chemotaxis. Proc Natl Acad Sci U S A. 2014 Jul 29. pii: 201322291. [Epub ahead of print]
[2] Zatulovskiy E, Tyson RA, Bretschneider T, Kay RR. Bleb-driven chemotaxis of Dictyostelium cells. J Cell Biol. 204(6):1027-1044, 2014.
Journal information: Proceedings of the National Academy of Sciences
Provided by University of Warwick