Highly efficient nanoparticles could bring down the cost of fuel cells
(Phys.org) —Fuel cells are a promising, non-polluting way to power cars, but their platinum catalysts are so expensive that there's no way current technology could be economically scaled up for widespread use. Now scientists at the Department of Energy's SLAC National Accelerator Laboratory and the Technical University of Denmark have developed an alternative that would use just one-fifth as much of the pricey metal.
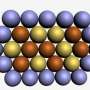
The new catalyst is a mixture of platinum and a second, cheaper element, yttrium, formed into nanoparticles whose size can be precisely controlled. Electron microscopy and X-ray studies show that yttrium atoms leach out of the surface of these particles, leaving a thin, dense, sturdy crust of platinum atoms to enthusiastically promote a key reaction in the fuel cell that converts oxygen molecules into water.
The results were published July 13 in Nature Chemistry.
"We now have proof of principle that these nanoparticles work the way we had predicted," said report co-author Daniel Friebel, an associate staff scientist at SUNCAT Center for Interface Science and Catalysis, which is jointly run by SLAC and Stanford University. "The next step is to find a more efficient way to make these nanoparticles so they can be mass-produced."
Wanted: Cheaper Fuel Cells
All but a handful of today's electric vehicles run on batteries, which are heavy and can only store so much energy; that's why electric cars have a limited range. Fuel cells are an attractive alternative because they're small and light and could run on a tank full of hydrogen replenished at a fueling station. In addition, the car's exhaust would contain nothing but pure water.
But the catalyst that breaks down oxygen molecules in a fuel cell requires five to 10 times more platinum than the catalytic converter that scrubs pollutants from conventional engine exhaust. With the price of platinum nearly $1,500 an ounce, running the world's automotive fleet on fuel cells would be prohibitively expensive.
"The number one goal is to minimize how much platinum you use, and you can only realize that goal with nanoparticles," Friebel said. "That's because the catalytic reaction happens only at the surface of the material; and the smaller the particle, the larger the surface area it has with respect to its interior volume."
But the smaller the particles, he said, the more unstable they become. Scientists have combined platinum with other elements, such as nickel, to make catalysts that initially outperformed pure platinum, but later fell behind as the non-platinum part of the alloy corroded away.
About five years ago, SUNCAT Director Jens Nørskov, a theorist who was then at Technical University of Denmark (DTU), and his coworkers suggested that a mixture of platinum and yttrium might do the trick. This seemed like an odd choice; yttrium likes to react with oxygen, which does not bode well for its stability, and the alloy would prove difficult to synthesize. But initial samples made by a company in Germany turned out to be both stable and a decent catalyst.
Testing an Unlikely Combo
To turn the samples into nanoparticles, researchers at DTU bombarded the alloy with argon ions in a vacuum chamber. This knocked out atoms of platinum and yttrium, which cooled and stuck together to form nanoparticles. The scientists sorted the particles by size and discovered that some of the larger ones – about 9 nanometers in diameter – had the best catalytic activity, five times better than today's pure platinum catalysts.
Then the scientists examined the nanoparticles with X-ray beams at SLAC's Stanford Synchrotron Radiation Lightsource, a DOE Office of Science user facility, to find out what made them so active. They found that the larger yttrium atoms had escaped from the surfaces of the particles, leaving a surface crust in which the smaller platinum atoms packed together more tightly than usual in a very stable configuration.
For a commercial catalyst, Friebel said, the team will need to find a more efficient way to make the nanoparticles. They'll also see if they can tune the density of the platinum crust so it will perform its tasks of bond-breaking and bond-making to convert oxygen into water even faster.
The 15-member research team included Patricia Hernandez-Fernandez, Ifan E.L Stephens and Ib Chorkendorff at DTU and Anders Nilsson of SUNCAT. Support for this research came from the Danish Ministry of Science, the Danish National Research Foundation, the A.P. Møller and Chastine Mc-Kinney Møller Foundation, the Interdisciplinary Center for Electron Microscopy at EPFL and the DOE Office of Science.
More information: "Mass-selected nanoparticles of PtxY as model catalysts for oxygen electroreduction." Patricia Hernandez-Fernandez,et al. Nature Chemistry (2014) DOI: 10.1038/nchem.2001. Received 03 January 2014 Accepted 10 June 2014 Published online 13 July 2014
Journal information: Nature Chemistry
Provided by SLAC National Accelerator Laboratory