Superconducting secrets solved after 30 years
(Phys.org) —A breakthrough has been made in identifying the origin of superconductivity in high-temperature superconductors, which has puzzled researchers for the past three decades.
Harnessing the enormous technological potential of high-temperature superconductors – which could be used in lossless electrical grids, next-generation supercomputers and levitating trains – could be much more straightforward in future, as the origin of superconductivity in these materials has finally been identified.
Superconductors, materials which can carry electric current with zero resistance, could be used in a huge range of applications, but a lack of understanding about where their properties originate from has meant that the process of identifying new materials has been rather haphazard.
Researchers from the University of Cambridge have found that ripples of electrons, known as charge density waves or charge order, create twisted 'pockets' of electrons in these materials, from which superconductivity emerges. The results are published in the June 15th issue of the journal Nature.
Low-temperature, or conventional, superconductors were first identified in the early 20th century, but they need to be cooled close to absolute zero (zero degrees on the Kelvin scale, or -273 degrees Celsius) before they start to display superconductivity. So-called high-temperature superconductors however, can display the same properties at temperatures up to 138 Kelvin (-135 degrees Celsius), making them much more suitable for practical applications.
Since they were first identified in the mid-1980s, the process of discovering new high-temperature superconductors could be best described as random. While researchers have identified the ingredients that make for a good low-temperature superconductor, high-temperature superconductors have been more reluctant to give up their secrets.
In a superconductor, as in any electronic device, current is carried via the charge on an electron. What is different about superconductors is that the electrons travel in tightly bound pairs. When travelling on their own, electrons tend to bump into each other, resulting in a loss of energy. But when paired up, the electrons move smoothly through a superconductor's structure, which is why superconductors can carry current with no resistance. As long as the temperature is kept sufficiently low, the electron pairs will keep moving through the superconductor indefinitely.
Key to conventional superconductors are the interactions of electrons with the lattice structure of the material. These interactions generate a type of 'glue' which holds the electrons together. The strength of the glue is directly related to the strength of the superconductor, and when the superconductor is exposed to an increase in temperature or magnetic field strength, the glue is weakened, the electron pairs break apart and superconductivity is lost.
"One of the problems with high-temperature superconductors is that we don't know how to find new ones, because we don't actually know what the ingredients are that are responsible for creating high-temperature superconductivity in the first place," said Dr Suchitra Sebastian of the Cavendish Laboratory, lead author of the paper. "We know there's some sort of glue which causes the electrons to pair up, but we don't know what that glue is."
In order to decode what makes high-temperature superconductors tick, the researchers worked backwards: by determining what properties the materials have in their normal, non-superconducting state, they might be able to figure out what was causing superconductivity.
"We're trying to understand what sorts of interactions were happening in the material before the electrons paired up, because one of those interactions must be responsible for creating the glue," said Dr Sebastian. "Once the electrons are already paired up, it's hard to know what made them pair up. But if we can break the pairs apart, then we can see what the electrons are doing and hopefully understand where the superconductivity came from."
Superconductivity tends to override other properties. For example, if in its normal state a superconductor was a magnet, suppressing that magnetism has been found to result in superconductivity. "So by determining the normal state of a superconductor, it would make the process of identifying new ones much less random, as we'd know what sorts of materials to be looking for in the first place," said Dr Sebastian.
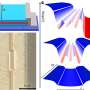
Working with extremely strong magnetic fields, the researchers were able to kill the superconducting effect in cuprates - thin sheets of copper and oxygen separated by more complex types of atoms.
Previous attempts to determine the origins of superconductivity by determining the normal state have used temperature instead of magnetic field to break the electron pairs apart, which has led to inconclusive results.
As cuprates are such good superconductors, it took the strongest magnetic fields in the world – 100 Tesla, or roughly one million times stronger than the Earth's magnetic field – in order to suppress their superconducting properties.
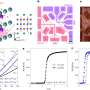
These experiments were finally able to solve the mystery surrounding the origin of pockets of electrons in the normal state that pair to create superconductivity. It was previously widely held that electron pockets were located in the region of strongest superconductivity. Instead, the present experiments using strong magnetic fields revealed a peculiar undulating twisted pocket geometry -similar to Jenga bricks where each layer goes in a different direction to the one above or beneath it.
These results pinpointed the pocket locations to be where superconductivity is weakest, and their origin to be ripples of electrons known as charge density waves, or charge order. It is this normal state that is overridden to yield superconductivity in the family of cuprate superconductors studied.
"By identifying other materials which have similar properties, hopefully it will help us find new superconductors at higher and higher temperatures, even perhaps materials which are superconductors at room temperature, which would open up a huge range of applications," said Dr Sebastian.
More information: "Normal-state nodal electronic structure in underdoped high-Tc copper oxides." Suchitra E. Sebastian, et al. Nature (2014) DOI: 10.1038/nature13326. Received 12 January 2014 Accepted 02 April 2014 Published online 15 June 2014
Journal information: Nature
Provided by University of Cambridge