Tricking the uncertainty principle
(Phys.org) —Caltech researchers have found a way to make measurements that go beyond the limits imposed by quantum physics.
Today, we are capable of measuring the position of an object with unprecedented accuracy, but quantum physics and the Heisenberg uncertainty principle place fundamental limits on our ability to measure. Noise that arises as a result of the quantum nature of the fields used to make those measurements imposes what is called the "standard quantum limit." This same limit influences both the ultrasensitive measurements in nanoscale devices and the kilometer-scale gravitational wave detector at LIGO. Because of this troublesome background noise, we can never know an object's exact location, but a recent study provides a solution for rerouting some of that noise away from the measurement.
The findings were published online in the May 15 issue of Science Express.
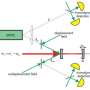
"If you want to know where something is, you have to scatter something off of it," explains Professor of Applied Physics Keith Schwab, who led the study. "For example, if you shine light at an object, the photons that scatter off provide information about the object. But the photons don't all hit and scatter at the same time, and the random pattern of scattering creates quantum fluctuations"—that is, noise. "If you shine more light, you have increased sensitivity, but you also have more noise. Here we were looking for a way to beat the uncertainty principle—to increase sensitivity but not noise."
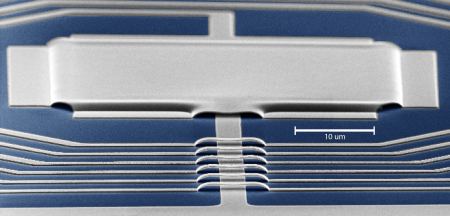
Schwab and his colleagues began by developing a way to actually detect the noise produced during the scattering of microwaves—electromagnetic radiation that has a wavelength longer than that of visible light. To do this, they delivered microwaves of a specific frequency to a superconducting electronic circuit, or resonator, that vibrates at 5 gigahertz—or 5 billion times per second. The electronic circuit was then coupled to a mechanical device formed of two metal plates that vibrate at around 4 megahertz—or 4 million times per second. The researchers observed that the quantum noise of the microwave field, due to the impact of individual photons, made the mechanical device shake randomly with an amplitude of 10-15 meters, about the diameter of a proton.
"Our mechanical device is a tiny square of aluminum—only 40 microns long, or about the diameter of a hair. We think of quantum mechanics as a good description for the behaviors of atoms and electrons and protons and all of that, but normally you don't think of these sorts of quantum effects manifesting themselves on somewhat macroscopic objects," Schwab says. "This is a physical manifestation of the uncertainty principle, seen in single photons impacting a somewhat macroscopic thing."
Once the researchers had a reliable mechanism for detecting the forces generated by the quantum fluctuations of microwaves on a macroscopic object, they could modify their electronic resonator, mechanical device, and mathematical approach to exclude the noise of the position and motion of the vibrating metal plates from their measurement.
The experiment shows that a) the noise is present and can be picked up by a detector, and b) it can be pushed to someplace that won't affect the measurement. "It's a way of tricking the uncertainty principle so that you can dial up the sensitivity of a detector without increasing the noise," Schwab says.
Although this experiment is mostly a fundamental exploration of the quantum nature of microwaves in mechanical devices, Schwab says that this line of research could one day lead to the observation of quantum mechanical effects in much larger mechanical structures. And that, he notes, could allow the demonstration of strange quantum mechanical properties like superposition and entanglement in large objects—for example, allowing a macroscopic object to exist in two places at once.
"Subatomic particles act in quantum ways—they have a wave-like nature—and so can atoms, and so can whole molecules since they're collections of atoms," Schwab says. "So the question then is: Can you make bigger and bigger objects behave in these weird wave-like ways? Why not? Right now we're just trying to figure out where the boundary of quantum physics is, but you never know."
This work was published in an article titled "Mechanically Detecting and Avoiding the Quantum Fluctuations of a Microwave Field."
Journal information: Science Express
Provided by California Institute of Technology