Study finds missing piece of biogeochemical puzzle in aquifers

(Phys.org) —A study published today in Science by researchers from the U.S. Department of Energy's Argonne National Laboratory may dramatically shift our understanding of the complex dance of microbes and minerals that takes place in aquifers deep underground. This dance affects groundwater quality, the fate of contaminants in the ground and the emerging science of carbon sequestration.
Deep underground, microbes don't have much access to oxygen. So they have evolved ways to breathe other elements, including solid minerals like iron and sulfur.
The part that interests scientists is that when the microbes breathe solid iron and sulfur, they transform them into highly reactive dissolved ions that are then much more likely to interact with other minerals and dissolved materials in the aquifer. This process can slowly but steadily make dramatic changes to the makeup of the rock, soil and water.
"That means that how these microbes breathe affects what happens to pollutants—whether they travel or stay put—as well as groundwater quality," said Ted Flynn, a scientist from Argonne and the Computation Institute at the University of Chicago and the lead author of the study.
About a fifth of the world's population relies on groundwater from aquifers for their drinking water supply, and many more depend on the crops watered by aquifers.
For decades, scientists thought that when iron was present in these types of deep aquifers, microbes who can breathe it would out-compete those who cannot. There's an accepted hierarchy of what microbes prefer to breathe, according to how much energy each reaction can theoretically yield. (Oxygen is considered the best overall, but it is rarely found deep below the surface.)
According to these calculations, of the elements that do show up in these aquifers, breathing iron theoretically provides the most energy to microbes. And iron is frequently among the most abundant minerals in many aquifers, while solid sulfur is almost always absent.
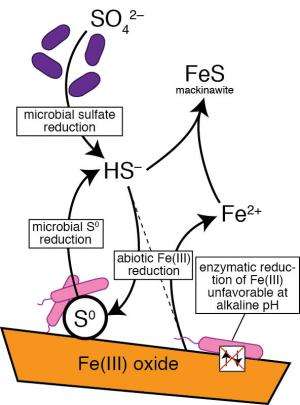
But something didn't add up right. A lot of the microorganisms had equipment to breathe both iron and sulfur. This requires two completely different enzymatic mechanisms, and it's evolutionarily expensive for microbes to keep the genes necessary to carry out both processes. Why would they bother, if sulfur was so rarely involved?
The team decided to redo the energy calculations assuming an alkaline environment—"Older and deeper aquifers tend to be more alkaline than pH-neutral surface waters," said Argonne coauthor Ken Kemner—and found that in alkaline environments, it gets harder and harder to get energy out of iron.
"Breathing sulfur, on the other hand, becomes even more favorable in alkaline conditions," Flynn said.
The team reinforced this hypothesis in the lab with bacteria under simulated aquifer conditions. The bacteria, Shewanella oneidensis, can normally breathe both iron and sulfur. When the pH got as high as 9, however, it could breathe sulfur, but not iron.
There was still the question of where microorganisms like Shewanella could find sulfur in their native habitat, where it appeared to be scarce.
The answer came from another group of microorganisms that breathe a different, soluble form of sulfur called sulfate, which is commonly found in groundwater alongside iron minerals. These microbes exhale sulfide, which reacts with iron minerals to form solid sulfur and reactive iron. The team believes this sulfur is used up almost immediately by Shewanella and its relatives.

"This explains why we don't see much sulfur at any fixed point in time, but the amount of energy cycling through it could be huge," Kemner said.
Indeed, when the team put iron-breathing bacteria in a highly alkaline lab environment without any sulfur, the bacteria did not produce any reduced iron.
"This hypothesis runs counter to the prevailing theory, in which microorganisms compete, survival-of-the-fittest style, and one type of organism comes out dominant," Flynn said. Rather, the iron-breathing and the sulfate-breathing microbes depend on each other to survive.
Understanding this complex interplay is particularly important for sequestering carbon. The idea is that in order to keep harmful carbon dioxide out of the atmosphere, we would compress and inject it into deep underground aquifers. In theory, the carbon would react with iron and other compounds, locking it into solid minerals that wouldn't seep to the surface.
Iron is one of the major players in this scenario, and it must be in its reactive state for carbon to interact with it to form a solid mineral. Microorganisms are essential in making all that reactive iron. Therefore, understanding that sulfur—and the microbe junkies who depend on it—plays a role in this process is a significant chunk of the puzzle that has been missing until now.
More information: The study, "Sulfur-Mediated Electron Shuttling During Bacterial Iron Reduction," appears online today in the May 1 edition of Science Express and will be published in Science at the end of the month. Other authors on the study were Argonne scientists Bhoopesh Mishra (also of the Illinois Institute of Technology) and Edward O'Loughlin and Georgia Tech scientist Thomas DiChristina.
Journal information: Science , Science Express
Provided by Argonne National Laboratory


















