Making graphene in your kitchen
Graphene has been touted as a wonder material—the world's thinnest substance, but super-strong. Now scientists say it is so easy to make you could produce some in your kitchen.
Thus far, graphene has proven elusively hard to manufacture on an industrial scale. Methods have required a choice between high quality graphene in small quantities, or large batches with defects.
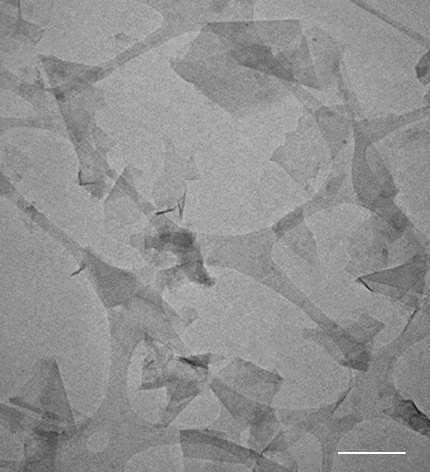
A team from England and Ireland, however, reported on Sunday they had used a blender to make microscopic sheets of graphene.
They placed powdered graphite, the stuff from which pencil lead is made, into a container with an "exfoliating liquid", and then mixed at high speed.
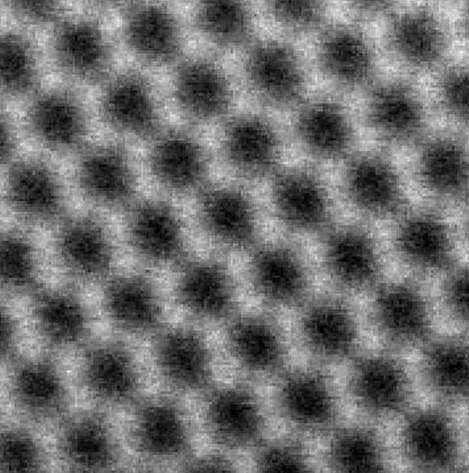
The result is miniscule sheets of graphene, each about a nanometre (a billionth of a metre) thick and 100 nanometres long, suspended in a liquid.
The force generated by the rotating blades separated the graphite into graphene layers without damaging their two-dimensional structure.
"We developed a new way of making graphene sheets," Trinity College Dublin chemical physics professor Jonathan Coleman, who co-authored the study in the journal Nature Materials, told AFP.
"This method gives lots of graphene with no defects."
The team used industrial equipment called shear mixers, but successfully repeated the experiment with a kitchen blender.

The liquid so produced can be spread onto surfaces as films of graphene sheets, like paint, or mixed with plastics to produce reinforced, composite materials.
"In the lab, we produced grams. However, when scaled up, tonnes will be produced," said Coleman.
Graphene is the world's thinnest substance, transparent but stronger than steel—a conductive super-material made of carbon just one atom thick.
There is a surge of interest in it to replace semiconductors in next-generation computers, touch screens, batteries and solar cells.
Graphene was aired as a theoretical substance in 1947. But for decades, physicists thought it would be impossible to isolate, as such thin crystalline sheets were bound to be unstable.
The problem was resolved in 2004 by a pair of scientists who used ordinary sticky tape to lift a layer from a piece of graphite.
That layer was itself pulled apart using more tape, and the process repeated until just the thinnest of layers remained—a graphene sheet.
Coleman said a company that sponsored the study has applied for a patent on the new method.
More information: Scalable production of large quantities of defect-free few-layer graphene by shear exfoliation in liquids, Nature Materials (2014) dx.doi.org/10.1038/nmat3944
Journal information: Nature Materials
© 2014 AFP




















