Chemical transformation yields surface-bound microstructures that repel oil- and water-based contaminants
Natural surfaces that repel water, such as lotus leaves or butterfly wings, often have a rough, microscale texture that traps air beneath the liquid droplet. By mimicking these biological structures, researchers have developed 'superhydrophobic' coatings that are highly resistant to wetting. One trick unknown to nature, however, is the ability to repel hydrocarbon-based oils that have much lower surface tension than water and tend to spread out rather than bead up.
Jia Min Chin and co-workers from the A*STAR Institute of Materials Research and Engineering and A*STAR Institute of Bioengineering and Nanotechnology in Singapore have now discovered a simple procedure to synthesize 'omniphobic' interfaces that repel both oil and water using intricate, mushroom-shaped, metal–organic crystal frameworks.
Recent efforts toward omniphobic surfaces have focused on producing reentrant microscale textures, which have curved shapes that inherently retain air pockets. These structures prevent oil from wetting the surface and stabilize the beaded droplet state. Currently, complicated and labor-intensive lithographic fabrication techniques are needed to generate such textures.
Chin and co-workers investigated a 'bottom-up' strategy to synthesize omniphobic films using metal–organic frameworks (MOFs)—compounds that connect metal ions into multidimensional structures using hydrocarbon-based linkages. Previous studies have shown that an aluminum-containing MOF, known as NH2-MIL-53(Al), can controllably form micro- and nanoscale rods and needles. The team suspected that suitable synthetic conditions could yield spontaneous needle growth upward from a substrate, forming a micro-rough surface with numerous trapped air pockets.
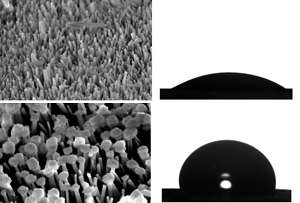
To achieve this, the researchers mixed their MOF precursor with an aluminum oxide membrane and applied 'hydrothermal' high temperature–high pressure aqueous reaction conditions. This resulted in perpendicularly aligned needles on both sides of the membrane. Next, the team faced the challenge of transforming the needles into curved textures suitable for repelling oil. After many attempts, they spotted an important clue—the modified membranes 'floated' on top of aqueous surfaces due to their superhydrophobic nature.
Chin and her team exploited this floating effect by suspending the microneedle-covered membrane in an aqueous solution of the MOF precursor. Additional MOF growth occurred only on the wetted tips of the needles, expanding the crystalline stems into mushroom-like caps (see image). By controlling the reaction time to generate a targeted cap size, the researchers' omniphobic surface successfully repelled long-chain hydrocarbon oils.
Chin notes that this benchtop, chemical process produces results previously limited to facilities with expensive, high-tech equipment. "Our aim was to develop simple techniques for fabricating interesting structures which are accessible to scientists around the world," she says.
More information: Tan, T. T. Y., Reithofer, M. R., Chen, E. Y., Menon, A. G., Hor, T. S. A., Xu, J. & Chin, J. M. "Tuning omniphobicity via morphological control of metal–organic framework functionalized surfaces." Journal of the American Chemical Society 135, 16272–16275 (2013). dx.doi.org/10.1021/ja407896m
Journal information: Journal of the American Chemical Society



















