Researchers describe oxygen's different shapes
(Phys.org) —Oxygen-16, one of the key elements of life on earth, is produced by a series of reactions inside of red giant stars. Now a team of physicists, including one from North Carolina State University, has revealed how the element's nuclear shape changes depending on its state, even though other attributes such as spin and parity don't appear to differ. Their findings may shed light on how oxygen is produced.
Carbon and oxygen are formed when helium burns inside of red giant stars. Carbon-12 forms when three helium-4 nuclei combine in a very specific way (called the triple alpha process), and oxygen-16 is the combination of a carbon-12 and another helium-4 nucleus.
Although physicists knew what oxygen-16 was made of, they were still puzzled by the fact that both the ground and first excited states of the element had zero spin and positive parity. A similar situation occurs in carbon-12 with the ground state and second zero-spin state known as the Hoyle state. At room temperature, only the ground state of oxygen-16 is seen due to the very cold temperature compared to nuclear energies. But the excited states of oxygen-16 become important for the helium-burning reactions inside stars.
"It's expected that oxygen-16 would have zero spin and positive parity as its ground state," says NC State physicist Dean Lee, team member and co-author of a paper describing the research. "What is unexpected is that the first excited state also has these qualities. It made us wonder what the real difference is between the states, which required looking at the structure of the eight protons and eight neutrons in oxygen-16. We had addressed a similar puzzle for the ground state and Hoyle state of carbon-12."
Lee, with colleagues Evgeny Epelbaum, Hermann Krebs, Timo Laehde, and Ulf-G. Meissner, had previously developed a new method for describing all the possible ways that protons and neutrons can bind with one another inside nuclei such as carbon-12 and the Hoyle state. They used an approach called "effective field theory" formulated on a complex numerical lattice that allows the researchers to run simulations that show how particles interact, and so reveal the structure of the nuclei.
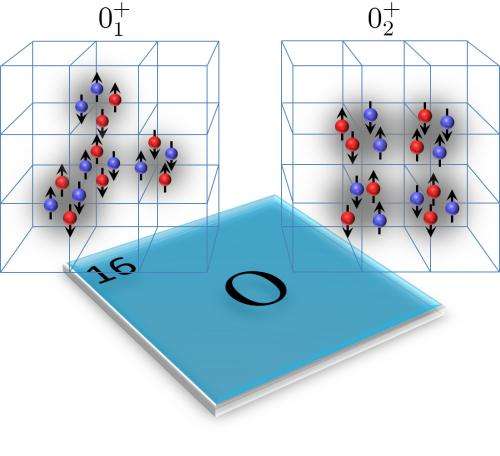
In this work, the same team plus Mississippi State physicist Gautam Rupak, found their lattice revealed that although both the ground and first excited states of oxygen-16 "look" the same in terms of spin and parity, they are in fact quite different structurally. In the ground state, the protons and neutrons are arranged in a tetrahedral configuration of four alpha clusters containing two protons and two neutrons each. For the first excited state, the alpha clusters are arranged in a square.
"The production of oxygen-16 from carbon-12 is still very poorly understood from both theoretical and experimental studies," Lee says. "These lattice simulations give us our first look at the structure of low-energy states of oxygen-16."
The results appear online March 12 in Physical Review Letters.
More information: "Ab Initio Calculation of the Spectrum and Structure of 16O" by Dean Lee et al Published: March 12, 2014 in Physical Review Letters.
Abstract:
We present ab initio lattice calculations of the low-energy even-parity states of 16O using chiral nuclear effective field theory. We find good agreement with the empirical energy spectrum, and with the electromagnetic properties and transition rates. For the ground state, we find that the nucleons are arranged in a tetrahedral configuration of alpha clusters. For the first excited spin-0 state, we find that the predominant structure is a square configuration of alpha clusters, with rotational excitations that include the first spin-2 state.
Journal information: Physical Review Letters
Provided by North Carolina State University