Toxic injection with elastic band
Bacteria have developed many different ways of smuggling their toxic cargo into cells. Tripartite Tc toxin complexes, which are used by bacteria like the plague pathogen Yersinia pestis and the insect pathogen Photorhabdus luminescens, are particularly unusual. Stefan Raunser from the Max Planck Institute of Molecular Physiology in Dortmund and his colleagues from the University of Freiburg have produced extremely accurate and detailed images of these "toxic injections"; they reveal from where the molecule complexes take the energy to penetrate the cell membrane. These proteins have potential applications in medicine and could, for example, selectively deposit drugs in cancer cells.
Photorhabdus luminescens lives in symbiosis with predatory roundworms. The bacterium waits in the intestine of the worm for it to attack an insect – at which point the bacterium comes into play, releasing Tc toxin complexes which quickly kill the insect. The roundworm and the bacterium then live together in the cadaver, before moving on to their next victim.
"Up until now, we didn't know much about the structure of Tc complexes," says Stefan Raunser. "All we had was a rough outline. Now we have images that even allow us to differentiate individual atoms!" With the aid of X-ray crystallography and electron cryomicroscopy the researchers were able to produce detailed images. "There's probably no other structure of a protein channel in which you can actually see the protein in the channel," says Raunser.
The Tc complexes are made up of the three sub-units TcA, TcB and TcC. TcA forms the channel that penetrates the cell membrane like an injection and deposits the toxic enzyme in the cell. Where the energy for this comes from, however, was previously unknown.
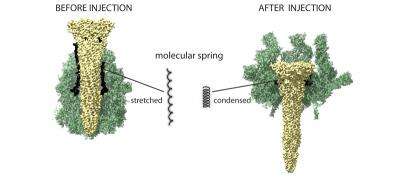
"TcA has a protein chain made up of 48 amino acids and this is stretched like an elastic band or a spring," says Raunser. If the band or spring contracts, energy is released that pushes the channel through the membrane. This mechanism is what sets the Tc complexes apart from all other known pore-forming injection proteins, such as those with diphtheria and anthrax bacteria.
The TcA sub-unit is moreover responsible for binding the complex to the host cell; for this purpose, it can avail of 20 receptor-binding domains. "We believe that four of these domains always surround one receptor, thereby increasing the strength of the binding," explains Raunser.
These receptor-binding domains can easily be removed or replaced with other domains. "This means that the Tc complexes can be directed to specific cells, for example cancer cells or other cells in the body." Once they have reached these cells, the Tc complexes can inject them with therapeutically effective molecules. Insect pathogenic Tc complexes could also be used as pesticides in the farming industry.
Provided by Max Planck Society


















