Protein structure: Peering into the transit pore
The lipid-rich membranes of cells are largely impermeable to proteins, but evolution has provided a way through – in the form of transmembrane tunnels. A new study shows in unmatched detail what happens as proteins pass through such a pore.
Every cell is surrounded by a surface membrane and contains internal compartments bounded by membranes. Almost one-third of all proteins synthesized in cells must pass through these membranes or be incorporated into them in order to fulfil their functions. However, the fat-rich nature of membranes makes it impossible for most proteins to percolate through them directly. Therefore, biological membranes contain so-called protein-conducting channels, molecular pores through which proteins can pass. "Structural investigations have already provided clues to how proteins are inserted into the membrane and then drawn through it like a length of thread to emerge on the other side," says Professor Roland Beckmann of the Gene Center of Ludwig-Maximilians-Universitaet (LMU) in Munich. "However, conclusive proof for these mechanisms has been lacking until now."Protein-conducting channels are known to be shaped like an hour-glass, consisting of two cones connected by a narrow central constriction. In the inactive form, the constriction is blocked by a plug that protrudes from the side-wall. Presumably, if a protein is to cross the membrane, the channel must be opened to provide a continuous aqueous environment for its passage. If, on the other hand, a protein is to be inserted within the membrane itself, it must emerge from a lateral opening within the tunnel.
Imaging the crucial transition
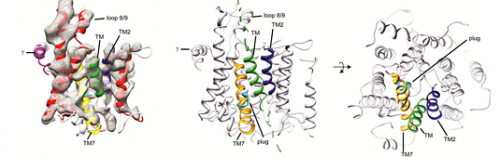
"Up until now, it had not been possible to characterize these structural transitions with the required spatial and temporal resolution," Beckmann says. Now he and his colleagues have, for the first time, succeeded in isolating transitional forms of the active channel, and elucidating their three-dimensional structures with the help of cryo-electron microscopy – at the unprecedented resolution of less than 1 nanometer. "This allowed us to determine the spatial conformations of the individual protein strands that make up the channel and to analyze how the channel behaves during its functional cycle," he explains.
Indeed, the researchers were even able to capture a snapshot of the complex at the moment when a protein leaves the channel to be incorporated into the cell membrane. "It turns out that there actually is a side-door within the channel, which opens to allow the protein to diffuse into the membrane," Beckmann says. Interestingly, as the lateral gate opens and the protein exits the channel into the membrane, the plug moves into the central constriction, blocking access to the outside and preventing diffusion of ions through the now empty channel. Surprisingly, proteins destined to cross the membrane do so without altering the position of the plug very much. Instead, an adjacent strand shifts slightly outward, widening the constriction sufficiently to let the protein through the length of the tunnel.
Beckmann and his team now hope to be able to increase the resolution of their snapshots still further. "Our goal is to achieve a resolution of less than 0.4 nm, in order to discern the interactions in molecular detail and understand the dynamic changes that take place in the structure of the channel," he says. In addition, the scientists want to image other membrane protein complexes – such as visual pigments – and analyze how a single chain of amino acids can function as a dynamically active membrane receptor.
More information: "Structures of the Sec61 complex engaged in nascent peptide translocation or membrane insertion." Marko Gogala, Thomas Becker, Birgitta Beatrix, Jean-Paul Armache, Clara Barrio-Garcia, Otto Berninghausen & Roland Beckmann, Nature 2014,DOI: 10.1038/nature12950. www.nature.com/nature/journal/ … ull/nature12950.html
Journal information: Nature
Provided by Ludwig Maximilian University of Munich