Ethanol blends carry hidden risk
(Phys.org) —Blending more ethanol into fuel to cut air pollution from vehicles carries a hidden risk that toxic or even explosive gases may find their way into buildings, according to researchers at Rice University.
Those problems would likely occur in buildings with cracked foundations that happen to be in the vicinity of fuel spills. Vapors that rise from contaminated groundwater can be sucked inside, according to Rice environmental engineer Pedro Alvarez. Once there, trapped pools of methane could ignite and toxic hydrocarbons could cause health woes, he said.
The timely warning comes as the United States works to stimulate the production and consumption of ethanol. The Rice study, detailed this week in the American Chemical Society journal Environmental Science and Technology, emerges as the Environmental Protection Agency (EPA) prepares technical guidance for higher ratios of ethanol in fuels.
"The safe distances (between buildings and groundwater) that the EPA are setting up are going to work well 95 percent of the time," said Alvarez, a member of the agency's Science Advisory Board. "But there's the 5 percent where things go wrong, and we need to be prepared for extreme events with low probability."
Computer simulations at Rice determined that fuel with 5 percent or less ethanol content does not rise to the level of concern, because small amounts of ethanol and benzene, a toxic, volatile hydrocarbon present in gasoline, degrade rapidly in the presence of oxygen. Methane produced when ethanol ferments is often degraded by methanotrophic bacteria, which also require oxygen.
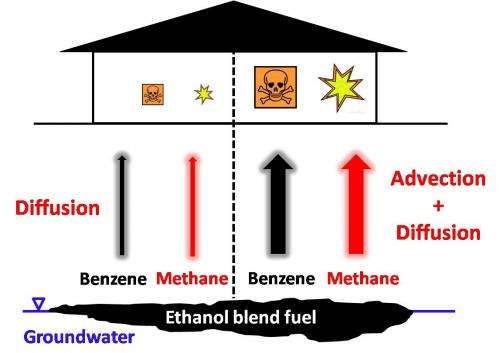
But fuel blends of 20 to 95 percent ethanol and gasoline, intended for "flex-fuel" vehicles, could increase the generation of methane. Ethanol and gasoline separate into distinct plumes as they spread underground from the site of a spill. As liquid ethanol degrades into gaseous methane, it expands, driving advective flow and forcing the gas outward – and upward. That could overwhelm natural attenuation and should prompt new thinking about how to manage vapor-intrusion risks, Alvarez said.
"We want the bacterial activity to eat these vapors before they reach us," Alvarez said. He said many factors, including shallow groundwater or soil with low permeability that is not easily ventilated, could stand in the way.
"The amount of oxygen allowed to diffuse in would determine the assimilative capacity of the soil and the degradation capability," he said. "The bacteria will be there, but they're not going to do you much good if they run out of oxygen. The problem is bacteria that eat the methane use up all the oxygen, and the ones you want to degrade benzene can't do their job because they don't have any oxygen left."
Benzene is a carcinogen. According to the federal Centers for Disease Control and Prevention, long-term exposure can harm bone marrow and cause a decrease in red blood cells, leading to anemia. It can also cause excessive bleeding and affect the immune system.
Alvarez said studies have assessed the amount of methane generated by spills, but none have directly addressed what happens when the highly flammable vapors rise into confined spaces, where they can accumulate. He said flux chambers have been used to measure methane in such spaces, but they don't account for building effects like typically lower interior pressure that would draw vapors in through cracked foundations.
So the Rice lab led by Alvarez, with the participation of researchers from Chevron, Shell and the University of Houston, programmed a three-dimensional vapor intrusion model to simulate the degradation, migration and intrusion pathways of methane and benzene under various site conditions. The program modeled a small building with a perimeter crack around the foundation, sitting in the center of an open field. The atmospheric pressure was assumed to be slightly less inside than outside.
The simulations determined that when there is no generation of methane from a plume, benzene would not be a problem—even for sources less than a meter below a foundation. But methane generation near a source significantly increased indoor concentrations of benzene; traces of the gas would be detected even when the source lay as much as 13 meters below a building.
Alvarez said the paper's lead author, Rice graduate student Jie Ma, has done extensive work to characterize bacterial activity at spill sites. "He figured out where the ones eating methane and consuming oxygen are most active. Most of them are concentrated in a very thin layer called the capillary fringe, where capillary forces suck the water up, above the saturated zone.
"It turns out this is a sweet spot where there's enough oxygen and moisture for the microbes to be happy, and it's close to high methane concentrations," Alvarez said. "They were present in several orders of magnitude higher than anywhere else. It's because of this biological filter that we rarely get explosions above the ground.
"That's the positive effect. The negative effect is that they're consuming the oxygen, and while they're saving us from explosions, they're allowing benzene to flow through. It's a trade-off."
Journal information: Environmental Science and Technology , Environmental Science & Technology
Provided by Rice University
"A numerical model investigation for potential methane explosion and benzene vapor intrusion associated with high-ethanol blend releases." Jie Ma, Hong Luo, George E Devaull, William G. Rixey, and Pedro J. J. Alvarez. Environmental Science & Technology. DOI: 10.1021/es403926k