Modifying one cell factor alters many others
Using a widely studied species of cyanobacterium, researchers from the RIKEN Center for Sustainable Resource Sciences have shown how difficult it is to alter the metabolism of a unicellular organism with the aim of producing a particular product without affecting other aspects of its functioning1.
Takashi Osanai and his team genetically engineered a strain of Synechocystis cyanobacterium to stimulate the breakdown of sugar and the production of biopolymers. Although the modification enhanced biopolymer production as intended, they found that the change also affected the size and shape of the cell, as well as photosynthesis, respiration and other aspects of its metabolism.
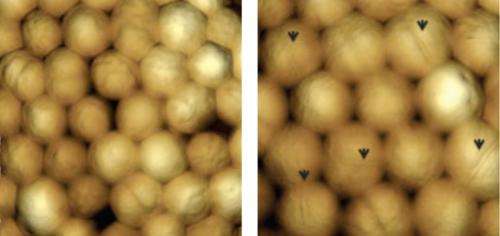
The genetic modification Osanai and his team engineered in Synechocystis involved overexpression of the sigE gene, which encodes a protein that regulates RNA synthesis known as a sigma factor. Under transmission electron and scanning probe microscopy, they found that the cell size of the engineered strain was larger than normal (Fig. 1) and there was evidence that the cell division process had been affected by the modification. Photosynthetic activity also tended to be higher when nitrogen was depleted but lower when nitrogen levels were normal and under high light conditions. Furthermore, hydrogen production increased at low oxygen levels. Several regulatory proteins were also seen to change with the elevated levels of the sigma factor protein.
According to the researchers, the results suggest a close relationship between metabolism, photosynthesis, cell form and hydrogen production. Despite all these differences, however, the modified strain remained just as viable as the normal strain over a range of nitrogen levels.
Apart from demonstrating the challenge in isolating cell factor-related alterations, the work also highlights the potential of cyanobacteria for the production of hydrogen for plastics and renewable energy applications. "We succeeded in increasing the production of hydrogen using the modified cyanobacteria," Osanai says. "The hydrogen was produced using light energy and the cells were cultured with carbon dioxide as a carbon source. Thus, we could possibly use our cyanobacterial strain to produce renewable energy that could replace fossil fuels and even nuclear power."
At present, the amount of hydrogen produced by this modified cyanobacterium is quite low, but Osanai is focused on exploiting the opportunity. "We will now try to increase hydrogen productivity by additional genetic engineering," he says. "We will also try to increase the synthesis of bioplastics. A deeper understanding of the mechanism of the relationship between all the factors we have uncovered will be important."
More information: Osanai, T., Kuwahara, A., Iijima, H., Toyooka, K., Sato, M., Tanaka, K., Ikeuchi, M., Saito, K. and Hirai, M. Y. (2013), Pleiotropic effect of sigE over-expression on cell morphology, photosynthesis and hydrogen production in Synechocystis sp. PCC 6803. The Plant Journal, 76: 456–465. doi: 10.1111/tpj.12310
Journal information: The Plant Journal
Provided by RIKEN