Researchers capture speedy chemical reaction in mid-stride
In synthetic chemistry, making the best possible use of the needed ingredients is key to optimizing high-quality production at the lowest possible cost.
The element rhodium is a powerful catalyst—a driver of chemical reactions—but is also one of the rarest and most expensive. In addition to its common use in vehicle catalytic converters, rhodium is also used in combination with other metals to efficiently drive a wide range of useful chemical reactions.
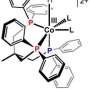
Chemists' efforts to study the inner workings of dirhodium metal complex reactions have been hindered by their extreme efficiency and speed, reacting at about 300 times per second. Now, a team of scientists led by University of Wisconsin–Madison chemistry professor John Berry reports an advance that freezes one step of the process long enough to offer researchers a glimpse into the finer mechanism.
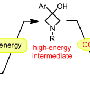
Chemical reactions pass through a series of steps from starting material to end product, with intermediate chemical structures formed at each step. The nature of those "part-way" compounds—called intermediates—can tell chemists a great deal about the processes and their efficiency.
However, intermediates normally exist for a second or less before moving to the next step in the reaction, making them extremely difficult to study. The new paper, appearing in this week's issue of Science Express (Sept. 12), describes the isolation and characterization of an intermediate that is stable for hours at 0 degrees Celsius.
"We've provided the first solid fundamental data on these compounds," said Berry, who led the effort to synthesize the stable version of a normally short-lived molecule. "People have thought about it for forty years, but this is the first time that we can actually see it and say this is definitely what's going on."
Berry and UW–Madison graduate student Katherine Kornecki used computational models to predict how the intermediate molecules might be trapped. From those predictions, they were able to identify a suitable dirhodium complex and starting material with the properties needed to stabilize the intermediate compound long enough to study it further.
Formation of the reactive intermediate is visible as the green starting material changes to an ocean blue color that faded over time. Ultraviolet-visible spectrometry showed the formation of a new molecule, and Berry rallied the help of collaborators to make sure they were actually capturing the desired intermediate.
Huw Davies from Emory University provided a starting material that allowed characterization of the compound by vibrational spectroscopy and nuclear magnetic resonance (NMR). Jochen Autschbach from the University of Buffalo used density function theory to predict the NMR features of the compound, and Kyle Lancaster from Cornell University elucidated the compound's structure using a series of X-ray absorption spectroscopy experiments.
"This paper is a wonderful example of how big challenges in chemistry can be solved by employing a multidisciplinary, collaborative approach," says Davies, professor of organic chemistry at Emory University and director of the Center for Selective C-H Functionalization.
In addition to providing evidence of an intermediate previously known only on paper, the finding opens new avenues for the field of catalysis. "Now that we can make the intermediate, we can further explore its reactivity. We can try reactions with substrates that nobody has ever thought of before," Berry says.
Journal information: Science
Provided by University of Wisconsin-Madison