July 8, 2013 report
Researchers perform first direct measurement of Van der Waals force
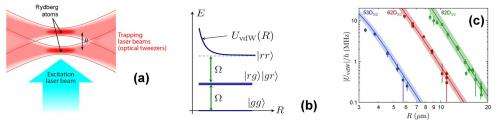
(Phys.org) —Researchers working at the French National Center for Scientific Research have for the first time, directly measured the Van der Waals force between two atoms. In their paper published in the journal Physical Review Letters, the team describes how they used lasers to hold two atoms steady and a third laser to measure the Van der Waals force between them.
The weak force between atoms, named after its discoverer Johannes Diderik van der Waals is evident in the behavior of many materials—it's what keeps most gas molecules together. Scientists have also discovered that it's also what allows a gecko's toes to stick to a smooth wall. But, because the weak force is only apparent when atoms are very close together, scientists have until now been unable to measure it directly.
In this new effort, the research team chose to use Rydberg atoms as part of their study. Such atoms are large and one of their electrons has a highly charged state. This makes them a good candidate for attempting to measure the Van der Waals force—they have more force between them than most other atom pairs and because of that can be measured at longer distances.
They started by firing a pair of lasers at twin Rydberg atoms. Doing so held them steady. Next, they fired a third laser at the two atoms causing them to oscillate at a desired frequency. By measuring the oscillations, the researchers were able to work out mathematically the Van der Waals force between them. More specifically, the researchers measured oscillations between ground and excited states, noting that the distance between the two atoms at the time of measurement was key—too close and the excitation of one of the atoms overwhelmed the other—too far and the force between the atoms became too weak to measure. Using the third laser as optic tweezers allowed for adjusting the distance between the two atoms, which ultimately led to just the right distance for measurement.
The team also noted that the technique used for measuring the Van der Waals force also led to the oscillating atoms evolving to a fully coherent state. This means the technique could be used to create quantum logic gates, which might prove useful in creating a quantum computer.
More information: Direct Measurement of the van der Waals Interaction between Two Rydberg Atoms, Phys. Rev. Lett. 110, 263201 (2013) prl.aps.org/abstract/PRL/v110/i26/e263201
Abstract
We report the direct measurement of the van der Waals interaction between two isolated, single Rydberg atoms separated by a controlled distance of a few micrometers. Working in a regime where the single-atom Rabi frequency for excitation to the Rydberg state is comparable to the interaction, we observe partial Rydberg blockade, whereby the time-dependent populations of the various two-atom states exhibit coherent oscillations with several frequencies. Quantitative comparison of the data with a simple model based on the optical Bloch equations allows us to extract the van der Waals energy, and observe its characteristic C6/R6 dependence. The measured C6 coefficients agree well with ab initio calculations, and we observe their dramatic increase with the principal quantum number n of the Rydberg state.
Journal information: Physical Review Letters
© 2013 Phys.org











.jpg)







