Test to improve stem cell safety
CSIRO scientists have developed a test to identify unsafe stem cells. It is the first safety test specifically for human induced pluripotent stem cells (iPS) – as published today in the international journal Stem Cells.
The breakthrough is a significant step in improving the quality of iPS cells and identifying unwanted cells that can form tumours. The test also determines how stable iPS cells are when grown in the lab. Dr Andrew Laslett and his team have spent the last five years working on the project. The research has focused on comparing different types of iPS cells with human embryonic stem cells. iPS cells are now the most commonly used pluripotent stem cell type for research.
"The test we have developed allows us to easily identify unsafe iPS cells. Ensuring the safety of these cell lines is paramount and we hope this test will become a routine screen as part of developing safe and effective iPS-based cell therapies," says Dr Laslett.
Using their test method, Dr Laslett's team has shown that certain ways of making iPS cells carry more risks. When the standard technique is used, which relies on viruses to permanently change the DNA of a cell, unwanted tumours are more likely to form. In comparison, cells made using methods which do not alter cell DNA, do not form tumours.
Dr Laslett hopes the study and the new test method will help to raise the awareness and importance of stem cell safety and lead to improvements in quality control globally.
"It is widely accepted that iPS cells made using viruses should not be used for human treatment, but they can also be used in research to understand diseases and identify new drugs. Having the assurance of safe and stable cells in all situations should be a priority," says Dr Laslett.
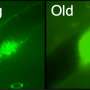
The test uses laser technology to identify proteins found on the surface of the cells. Based on the presence or absence of specific proteins the cells are then separated and monitored. Unsafe stem cell lines are easily identified because they form recognisable clusters of cells and the safe ones don't. This test could also be applied to assess the safety of the recently announced somatic cell nuclear transfer human embryonic stem cells.
Professor Martin Pera, Program Leader of Stem Cells Australia, said: "Although cell transplantation therapies based on iPS cells are being fast-tracked for testing in humans, there is still much debate in the scientific community over the potential hazards of this new technology."
"This important study provides a simple and powerful technique for assessing how safe stem cell lines are for use in patients," he adds.
More information: The paper, titled 'Identification of unsafe human induced pluripotent stem cell lines using a robust surrogate assay for pluripotency' is available on the Stem Cells website.
Journal information: Stem Cells
Provided by CSIRO