Researchers turn cement into metal
(Phys.org) —In a move that would make the Alchemists of King Arthur's time green with envy, scientists have unraveled the formula for turning liquid cement into liquid metal. This makes cement a semi-conductor and opens up its use in the profitable consumer electronics marketplace for thin films, protective coatings, and computer chips.
"This new material has lots of applications including as thin-film resistors used in liquid-crystal displays, basically the flat panel computer monitor that you are probably reading this from at the moment," said Chris Benmore, a physicist from the U.S. Department of Energy's (DOE) Argonne National Laboratory who worked with a team of scientists from Japan, Finland, and Germany to take the "magic" out of the cement-to-metal transformation. Benmore and Shinji Kohara from Japan Synchrotron Radiation Research Institute/SPring-8 led the research effort.
This change demonstrates a unique way to make metallic-glass material, which has positive attributes including better resistance to corrosion than traditional metal, less brittleness than traditional glass, conductivity, low energy loss in magnetic fields, and fluidity for ease of processing and molding. Previously only metals have been able to transition to a metallic-glass form. Cement does this by a process called electron trapping, a phenomena only previously seen in ammonia solutions. Understanding how cement joined this exclusive club opens the possibility of turning other solid normally insulating materials into room-temperature semiconductors.
"This phenomenon of trapping electrons and turning liquid cement into liquid metal was found recently, but not explained in detail until now," Benmore said. "Now that we know the conditions needed to create trapped electrons in materials we can develop and test other materials to find out if we can make them conduct electricity in this way."
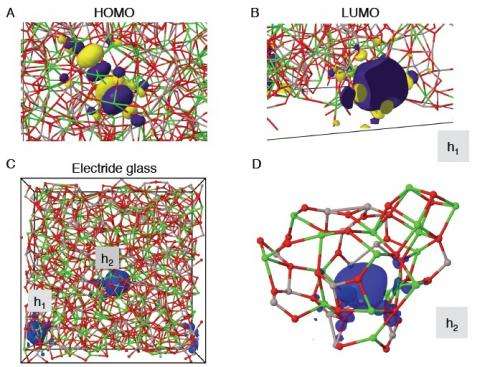
The results were reported May 27 in the journal the Proceedings of the National Academy of Sciences in the article "Network topology for the formation of solvated electrons in binary CaO–Al2O3 composition glasses".
The team of scientists studied mayenite, a component of alumina cement made of calcium and aluminum oxides. They melted it at temperatures of 2,000 degrees Celsius using an aerodynamic levitator with carbon dioxide laser beam heating. The material was processed in different atmospheres to control the way that oxygen bonds in the resulting glass. The levitator keeps the hot liquid from touching any container surfaces and forming crystals. This let the liquid cool into glassy state that can trap electrons in the way needed for electronic conduction. The levitation method was developed specifically for in-situ measurement at Argonne's Advanced Photon Source by a team led by Benmore.
The scientists discovered that the conductivity was created when the free electrons were "trapped" in the cage-like structures that form in the glass. The trapped of electrons provided a mechanism for conductivity similar to the mechanism that occurs in metals.
To uncover the details of this process, scientists combined several experimental techniques and analyzed them using a supercomputer. They confirmed the ideas in experiments using different X-ray techniques at Spring 8 in Japan combined with earlier measurements at the Intense Pulsed Neutron Source and the Advanced Photon Source.
More information: Network topology for the formation of solvated electrons in binary CaO–Al2O3 composition glasses, www.pnas.org/cgi/doi/10.1073/pnas.1300908110
Journal information: Proceedings of the National Academy of Sciences
Provided by Argonne National Laboratory












.jpg)







