Water splitting: Plants provide blueprint for cheap hydrogen production
(Phys.org) —The process by which plants convert energy from the sun's rays into chemical 'fuel' has inspired a new way of generating clean, cheap, renewable hydrogen power which could solve looming problems with the UK's energy infrastructure.
Hydrogen is a significant source of energy which can be burned to produce power with no negative impact on the environment, unlike power produced by burning fossil fuels. Hydrogen gas can be easily produced by splitting water into its constituent elements – hydrogen and oxygen.
Plants' powers of photosynthesis allow them to harness the energy of the sun to split water molecules into hydrogen and oxygen at separate times and at separate physical locations in the plant's structure.
By applying direct current to water via a positive and a negatively-charged electrode in a process known as electrolysis, scientists have long been able to break the bonds between hydrogen and oxygen, releasing them as gas.
Industrial processes to produce pure hydrogen from water require expensive equipment and rigorous oversight to ensure that the gases do not mix. Accidental mixing of the gases can lead to accelerated decay of materials involved in the process or even dangerously explosive mixtures.
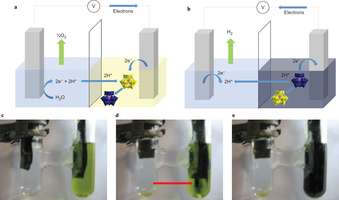
In a new paper in the journal Nature Chemistry published today (Monday 14 April), Professor Lee Cronin and Dr Mark Symes of the University of Glasgow outline how they have managed to replicate for the first time plants' ability to decouple the production of hydrogen and oxygen from water using what they call an electron-coupled proton buffer (ECPB).
Dr Symes: "What we have developed is a system for producing hydrogen on an industrial scale much more cheaply and safely than is currently possible. Currently much of the industrial production of hydrogen relies on reformation of fossil fuels, but if the electricity is provided via solar, wind or wave sources we can create an almost totally clean source of power.
"The ECPB is made from commercially-available phosphomolyb-dic acid. The properties of this material allow us to collect and store the protons and electrons which are generated when we oxidise water, to give oxygen as the only gaseous product. We can then use those stored protons and electrons to produce only hydrogen at a time of our choosing, allowing us to produce pure hydrogen gas on demand with none of the difficulties of the current electrolytic process where the two are unavoidably produced at the same time.
"Using a single precious metal electrode and an ECPB to generate hydrogen and oxygen from water would allow much more economically-viable large-scale generation of hydrogen than is currently possible."
Professor Cronin added: "One of the problems of generating electricity via renewable power is that the output either needs to be used immediately or stored. Using renewable power to produce hydrogen allows us to capture the electricity in a state which is easily stored and distributed and, when burned, creates no problems for the environment.
"In the next couple of decades we're likely to face significant problems because the infrastructure which allows the distribution of electricity across the country via power lines is ageing badly and will become increasingly less fit for purpose. There are currently no solid plans in place to source the billions of pounds it will cost to overhaul the system.
"However, the existing gas infrastructure which brings gas to homes across the country could just as easily carry hydrogen as it currently does methane. If we were to use renewable power to generate hydrogen using the cheaper, more efficient decoupled process we've created, the country could switch to hydrogen to generate our electrical power at home. It would also allow us to significantly reduce the country's carbon footprint."
More information: Professor Cronin's paper 'Decoupling Hydrogen and Oxygen Evolution During Electrolytic Water Splitting Using a Proton-Coupled Electron Buffer' is published in Nature Chemistry. www.nature.com/nchem/journal/v … full/nchem.1621.html
Journal information: Nature Chemistry
Provided by University of Glasgow