March 22, 2013 feature
Evidence of magnetic superatoms could open doors to new spin electronics
(Phys.org) —Scientists have found evidence for the existence of magnetic superatoms—small, compact clusters of atoms whose electrons occupy a set of orbitals around the entire cluster rather than around the individual atoms. If scientists can synthesize superatoms with magnetic properties, then one day they may use them to create new spin-dependent electronics.
The researchers, Xinxing Zhang, et al., from Johns Hopkins University in Baltimore, Maryland; Universitaet Konstanz in Konstanz, Germany; and Virginia Commonwealth University in Richmond, Virginia, have published their findings on magnetic superatoms in a recent issue of the Journal of the American Chemical Society.
To find evidence for the existence of stable, magnetic superatoms, the researchers probed the superatoms' orbitals, ground states, spin properties, and magnetic properties using first principles calculations and spectroscopy experiments.
Previous research has shown that, while the orbital shapes of superatoms resemble those of single atoms, electrons fill orbitals in superatoms differently than they fill those in single atoms. In single atoms, orbital filling follows Hund's rule, so that every orbital in a subshell is occupied with one electron before any orbital is doubly occupied. This approach generally leads to greater atomic stability.
However, superatom orbitals are filled differently due to spatial distortions, with the result being that superatoms are generally non-magnetic. One way to stabilize magnetic superatoms is to introduce an additional transition metal atom that can breed magnetism through its specific orbital configuration that hybridizes with the superatom and stabilizes the magnetic state.
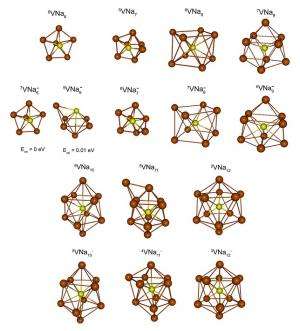
In the new study, the scientists showed that a non-magnetic cluster consisting of eight sodium atoms can be made magnetic by adding a vanadium atom to fill certain orbitals. The result is a stable magnetic superatom VNa8 that can be synthesized inside molecular beams.
"While making VNa8 species in molecular beams was not difficult, one should expect that making them in macroscopic quantities would be more challenging," coauthor Kit Bowen, a professor of chemical physics at Johns Hopkins University, told Phys.org.
The magnetic superatoms could be of interest for practical purposes if they can be supported on a substrate and used to generate spin-polarized electric currents. In this way, the magnetic superatoms could be used to build spin-dependent electronics.
"The new superatoms will have great potential in molecular electronic devices for spintronics and other applications," said coauthor Shiv Khanna, a physics professor at Virginia Commonwealth University. "In a recent work, my group has shown that a molecule made of two superatoms can lead to highly spin polarized currents. The minimal spin orbit would ensure that the electron spin maintains its direction for a longer time, which is important for applications in spintronics. We have also found that such devices can lead to high magneto resistance."
The researchers have future plans to continue exploring magnetic superatoms and what can be done with them.
"My group is now engaged in furthering our concept to propose different classes of such superatoms and the properties offered by each group," Khanna said. "On the other hand, we are talking with synthetic chemists to explore if we can make a material consisting of an assembly of magnetic superatomic building blocks."
More information: Xinxing Zhang, et al. "On the Existence of Designer Magnetic Superatoms." Journal of the American Chemical Society. DOI: 10.1021/ja400830z
Journal information: Journal of the American Chemical Society
Copyright 2013 Phys.org
All rights reserved. This material may not be published, broadcast, rewritten or redistributed in whole or part without the express written permission of Phys.org.