An Alchemist's Dream: Superatoms Mimic Elements
(PhysOrg.com) -- Dr. Will Castleman and his team have discovered clusters of atoms that mimic some of the properties of other elements. Called "superatoms," these clusters of atoms behave like a single "superatom" of a different species, and they may have implications as significant as the alchemists' search for gold.
Castleman's search was part of Pacific Northwest National Laboratory's Frontiers in Chemical Physics & Analysis Seminar Series. The series features academic, government, and industrial leaders who discuss novel ideas and advances in research and development.
Superatom clusters could serve as building blocks for new materials that are cheaper and more effective than materials currently being used as catalysts in chemical processing, and in the catalytic converters of automobiles. They may even have potential as new sources of energy.
The superatom concept was observed in studies involving aluminum clusters where, depending on the number of aluminum atoms constituting the cluster, reactivity patterns would emerge mimicking the chemistry associated with isolated elements. Identifying potential cluster species in the gas-phase as novel catalysts that could be used to assemble new materials with specific properties has been an ongoing research pursuit in the Castleman labs at Pennsylvania State University. Instrumental in these studies were Patrick Roach and Samuel Peppernick, both former members of Dr. Castleman's group and now postdoctoral researchers at PNNL.
Electron configurations key to mimicking phenomenon: Castleman and his team have shown that certain combinations of elemental atoms have electron configurations that mimic those of other elements. The researchers also showed that the atoms that have been identified so far in these mimicry events can be predicted simply by looking at the periodic table.
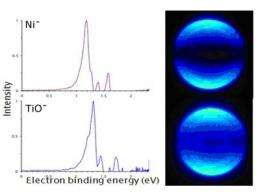
Initially, Dr. Castleman and his team observed chemical behavior in superatom clusters that reflected the behavior of a given element. To provide quantitative evidence, they examined the photoelectron behavior in the superatoms, detaching and analyzing the electrons of a given cluster of atoms to see what orbital the electrons were coming from.
"We started working with titanium monoxide (TiO) and much to our surprise we saw the TiO was isoelectronic (having very similar electronic configurations) with nickel," Castleman said. "This amazed us because we started seeing behaviors where TiO looked like nickel. We thought this must just be a chance happening."
But the team looked at other species, including zirconium oxide, which is isoelectronic with palladium, and tungsten carbide which is isoelectronic with palladium. "When we analyzed the behavior for each of these species, we saw a behavior that was amazingly similar. It looked as though TiO was a superatom of nickel."
Using the periodic table to predict behavior: With this discovery, the team returned to the periodic table to look at species with similar valence electrons. They found that superatoms mimicking other elements could be predicted by simple arithmetic. Titanium, for example, has four outer-shell electrons, atomic oxygen has six. So move six elements to the right of titanium and you're at nickel, whose 10 outer-shell electrons make it isoelectronic with titanium oxide. Assuming this finding must be a coincidence, Castleman and his team tried it with other atoms and found a definite pattern. Castleman said he doesn't know if the pattern will be repeated across the entire periodic table. Right now, he and his team are working through the transition-metal atoms.
So why use a different element if the actual element is available? First, the element mimic might be less expensive, as in the case of palladium, which, at $100 a gram, is used as a catalyst in jet engines. At two cents a gram, zirconium oxide would be a worthy substitute. Dr. Castleman and his team are pursing this through a U.S Air Force grant.
Another reason is that you might want some cluster that has different functionalities than the actual element. "It might behave like one particular element, but would do something else, like detect something or destroy something," Castleman said.
Provided by Pacific Northwest National Laboratory




















