February 28, 2013 report
Chemists discover a way to extract hydrogen from methanol at low temperature
(Phys.org)—A team of German and Italian chemists working at the University of Rostock has discovered a way to extract hydrogen from methanol at low temperatures and ambient pressure using a common catalyst. As they report in their paper published in the journal Nature, the team was able to use a ruthenium-based catalyst to extract hydrogen molecules from methanol without having to exert high temperatures or added pressure.
Methanol, best known as wood or "spirit" alcohol, occurs in small amounts in nature. More commonly however, it is created using a catalytic process involving hydrogen, carbon monoxide and carbon dioxide. It's most common use is as an ingredient in making other chemicals, such as formaldehyde. In this new research, the team became interested in methanol because of its possible use as a liquid carrier of hydrogen.
Over the past several years researchers have been looking for a way to store and transport hydrogen gas (for use in fuel cells to create electricity) in a way that is safe—most center around converting it to a liquid, a process that is difficult and requires a lot of energy. A better approach would be to use another liquid that has a lot of hydrogen in it and then to extract the hydrogen from it at the place where it is needed. One such liquid is methanol (12.6 percent hydrogen), but until now, extracting the hydrogen from it has required both high temperatures and pressure.
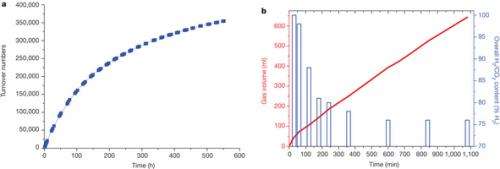
In this new research, the team used ruthenium as a catalyst along with an alkali to pull the molecules in methanol apart in a three step dehydrogenation process that resulted in the release of hydrogen gas (and carbon dioxide) at temperatures of 65–95 degrees Celsius at ambient air pressure. Furthermore the team found that they could produce three molecules of hydrogen for every one molecule of methanol used, proof that it was a highly efficient process.
Based on these results the team says it appears possible that methanol could be used as a liquid transport for hydrogen at some point in the future. There are some serious issues to be resolved before that could happen though, with the most glaring being capturing the carbon dioxide that is released during the process. Also, the process happens too slowly for use in a real time engine and needs to be made more efficient to reduce the amount of energy used overall.
More information: Low-temperature aqueous-phase methanol dehydrogenation to hydrogen and carbon dioxide, Nature (2013) doi:10.1038/nature11891
Abstract
Hydrogen produced from renewable resources is a promising potential source of clean energy. With the help of low-temperature proton-exchange membrane fuel cells, molecular hydrogen can be converted efficiently to produce electricity. The implementation of sustainable hydrogen production and subsequent hydrogen conversion to energy is called "hydrogen economy". Unfortunately, its physical properties make the transport and handling of hydrogen gas difficult. To overcome this, methanol can be used as a material for the storage of hydrogen, because it is a liquid at room temperature and contains 12.6 per cent hydrogen. However, the state-of-the-art method for the production of hydrogen from methanol (methanol reforming) is conducted at high temperatures (over 200 degrees Celsius) and high pressures (25–50 bar), which limits its potential applications. Here we describe an efficient low-temperature aqueous-phase methanol dehydrogenation process, which is facilitated by ruthenium complexes. Hydrogen generation by this method proceeds at 65–95 degrees Celsius and ambient pressure with excellent catalyst turnover frequencies (4,700 per hour) and turnover numbers (exceeding 350,000). This would make the delivery of hydrogen on mobile devices—and hence the use of methanol as a practical hydrogen carrier—feasible.
Journal information: Nature
© 2013 Phys.org