Engineering algae to make complex anti-cancer 'designer' drug
Biologists at UC San Diego have succeeded in genetically engineering algae to produce a complex and expensive human therapeutic drug used to treat cancer.
Their achievement, detailed in a paper in this week's early online issue of The Proceedings of the National Academy of Sciences, opens the door for making these and other "designer" proteins in larger quantities and much more cheaply than can now be made from mammalian cells.
"Because we can make the exact same drug in algae, we have the opportunity to drive down the price down dramatically," said Stephen Mayfield, a professor of biology at UC San Diego and director of the San Diego Center for Algae Biotechnology or SD-CAB, a consortium of research institutions that is also working to develop new biofuels from algae. Their method could even be used to make novel complex designer drugs that can't be produced in any other systems—drugs that could be used to treat cancer or other human diseases in new ways.
"You can't make these drugs in bacteria, because bacteria are incapable of folding these proteins into these complex, three-dimensional shapes," said Mayfield. "And you can't make these proteins in mammalian cells because the toxin would kill them."
The advance is the culmination of seven years of work in Mayfield's laboratory to demonstrate that Chlamydomonas reinhardtii, a green alga used widely in biology laboratories as a genetic model organism, can produce a wide range of human therapeutic proteins in greater quantity and more cheaply than bacteria or mammalian cells.
Mayfield and his colleagues achieved their first breakthrough five years ago when they demonstrated they could produce a mammalian serum amyloid protein in algae. The following year, they succeeded in getting algae to produce a human antibody protein. In 2010, they demonstrated that more complex proteins—human therapeutic drugs, such as human vascular endothelial growth factor, or VEGF, used to treat patients suffering from pulmonary emphysema—could be produced in algae.
Then in May of this year, Mayfield's group working with another team headed by Joseph Vinetz from UC San Diego's School of Medicine, engineered algae to produce an even more complex protein—a new kind of vaccine that, preliminary experiments suggest, could protect billions of people from malaria, one of the world's most prevalent and debilitating diseases.
"What the development of the malarial vaccine showed us was that algae could produce proteins that were really complex structures, containing lots of disulfide bonds that would still fold into the correct three-dimensional structures," said Mayfield. "Antibodies were the first sophisticated proteins we made. But the malarial vaccine is complex, with disulfide bonds that are pretty unusual. So once we made that, we were convinced we could make just about anything in algae."
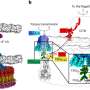
In their latest development, the scientists genetically engineered algae to produce a complex, three-dimensional protein with two "domains"—one of which contains an antibody, which can home in on and attach to a cancer cell and another domain that contains a toxin that kills the bound cancer cells. Such "fusion proteins" are presently created by pharmaceutical companies in a complex, two-step process by first developing the antibody domain in a Chinese hamster, or CHO, cell. The antibody is purified, then chemically attached to a toxin outside of the cell. Then the final protein is repurified.
"We have a two-fold advantage over that process," said Mayfield. "First, we make this as a single protein with the antibody and toxin domains fused together in a single gene, so we only have to purify it one time. And second, because we make this in algae rather than CHO cells, we get an enormous cost advantage on the production of the protein."
The fusion protein the researchers in his laboratory produced from algae is identical to one that is under development by pharmaceutical companies with a proposed cost of more than $100,000. This same protein could be produced in algae for a fraction of that price, they report in their paper. And the UCSD researchers—Miller Tran, Christina Van, Dan Barrera and Jack Bui at the UC San Diego Medical School—confirmed that the compound worked like the more expensive treatment: it homed in on cancer cells and inhibited the development of tumors in laboratory mice.
Mayfield said such a fusion protein could not have been produced in a mammalian CHO cell, because the toxin would have killed it. But because the protein was produced in the algae's chloroplasts—the part of algal and plant cells where photosynthesis takes place—it did not kill the algae.
"The protein was sequestered inside the chloroplast," Mayfield said. "And the chloroplast has different proteins from the rest of the cell, and these are not affected by the toxin. If the protein we made were to leak out of the chloroplast, it would have killed the cell. So it's amazing to think that not one molecule leaked out of the chloroplasts. There are literally thousands of copies of that protein inside the chloroplasts and not one of them leaked out."
Mayfield said producing this particular fusion protein was fairly straightforward because it involved fusing two domains—one to recognize and bind to cancer cells and another to kill them. But in the future, he suspects this same method could be used to engineer algae to produce more complex proteins with multiple domains.
"Can we string together four or five domains and produce a designer protein in algae with multiple functions that doesn't exist in nature? I think we can?" he added. "Suppose I want to couple a receptor protein with a series of activator proteins so that I could stimulate bone production or the production of neurons? At some point you can start thinking about medicine the same way we think about assembling a computer, combining different modules with specific purposes. We can produce a protein that has one domain that targets the kind of cell you want to impact, and another domain that specifies what you want the cell to do."
Journal information: Proceedings of the National Academy of Sciences
Provided by University of California - San Diego