A new phase in cellular communication
(Phys.org)—In many physical processes, substances undergo phase transitions, where they are transformed from one state (solid, liquid, or gas) to another. Wiskott-Aldrich Syndrome Proteins (WASP) function as intracellular signaling molecules, and one member of the family, N-WASP, interacts with two other proteins, forming a complex that plays an integral role in the regulation of the cell's internal scaffold. This interaction provides a system for investigation of phase transitions that result from multivalent protein-protein interactions. Utilizing the Biophysics Collaborative Access Team (Bio-CAT) synchrotron x-ray facility at the Advanced Photon Source, researchers investigated interactions between engineered multivalent substances. Understanding this research will be important in guiding future studies to further evaluate the role of phase transitions in biological systems.
The researchers in this study, from the University of Texas Southwestern Medical Center; the Illinois Institute of Technology; Louisiana State University; the University of California, Berkeley; and The Pennsylvania State University described the occurrence of sharp liquid-liquid-demixing phase separations corresponding to transformation between small complexes and large polymers at the molecular level, leading to the production of micrometer-diameter liquid droplets in aqueous solution. This phenomenon was observed for several different protein systems, suggesting that it may be a general property of multivalent macromolecules. In the N-WASP system, phosphorylation of one of its interacting proteins by a kinase enzyme controls the phase separation, demonstrating how this behavior could be regulated in vivo.
Phase transitions occur widely throughout nature, and involve transformation of a substance from one phase to another in response to changes in external conditions. One well-known example is that of evaporation, where liquid water is transformed to water vapor when temperature reaches a critical value (100 °C at 1 atmosphere pressure).
It has been known since the 1940s that interactions between multivalent (multiple unit) small molecules can lead to "sol-gel transitions," which involve sharp transformations between small assemblies and large polymer gels. In these processes, the sol-gel transition can occur when the concentration of the molecules reaches a critical value.
Just as differences in pressure can change the boiling point of water, differences in the physical properties of a multivalent molecule can change the critical concentration for a sol-gel transition. The polymer formed by these transitions can take on various physical forms, from liquid to solid, again depending on the properties of the molecules.
Multivalency has been investigated mostly in relation to extracellular substances binding to receptors on the cell surface. In this context, protein systems aggregate to form cross-linked networks, especially precipitates, but also liquid-like gels.
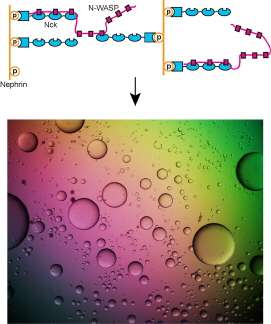
Many biological processes involve interactions between multivalent molecules, including intracellular signaling. Members of the WASP family of proteins act as intracellular signaling molecules in the regulation of the cellular scaffolding, or cytoskeleton. They are involved in transfer of signals from receptors on the cell surface to the inside of the cell, where they promote assembly of the cytoskeletal protein actin into long filaments. N-WASP is a member of this family with highest levels in the nervous system, and, together with its two protein partners (Nck and phosphorylated nephrin), represents a system for investigation of phase transitions that result from multivalent interactions.
This study aimed to investigate multivalency in relation to intracellular molecules, and in particular to determine whether these systems also experience sharp transformations to polymers. Utilizing small-angle x-ray scattering at the Bio-CAT 18-ID beamline to evaluate reactions, the researchers showed that interactions between synthetic, multivalent substances result in sharp liquid-liquid-demixing phase separations, in which two liquid substances separate to produce 1-50 µm diameter liquid droplets in aqueous solution (analogous to oil droplets in water). At the molecular level, this correlates with a transformation between small complexes and large polymers.
When they investigated N-WASP in this way, the researchers discovered that when it interacts with its two protein partners, a phase transition also occurs. This transformation is regulated by the degree of phosphorylation of nephrin, demonstrating how kinase enzymes could control this behavior of the system.
Further, the phase transition also increases the ability of N-WASP to promote formation of actin filaments, explaining why this behavior might be biologically important.
This study has provided new insights into how macroscopic structures (phase separated liquid droplets) can be generated from individual protein molecules. The work has also suggested why this behavior may be important in biological processes, including intracellular signaling.
The results of this study will help guide further research in biophysics, biochemistry, and cell biology on the assembly of proteins into large structures in the cell. Since multivalent systems are very common in nature, it seems possible that phase transitions may be involved in many aspects of biology through their ability to control spatial organization within cells, and regulate biochemical information transfer.
More information: Pilong Li, et al., Phase transitions in the assembly of multivalent signalling proteins. Nature 483, 336 (5 March 2012). DOI:10.1038/nature10879
Journal information: Nature
Provided by Argonne National Laboratory