Capping ligands as selectivity switches in hydrogenation reactions
The role of surface modification of nanoparticle catalysts in alkyne hydrogenation reactions was systematically investigated by the Center for Nanoscale Materials' NanoBio Interfaces Group working with researchers in Argonne's Chemical Sciences & Engineering and X-Ray Science divisions. The team explains the effect of surface ligands on the selectivity and activity of platinum and Co/Pt nanoparticles using experimental and computational approaches.
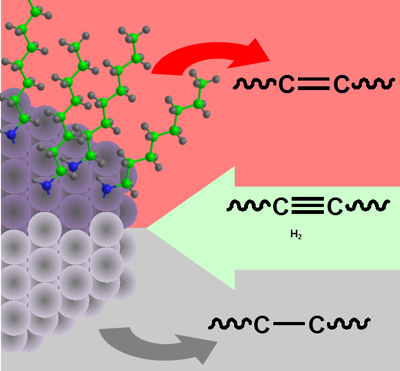
A tunable balance between the adsorption energetics of alkenes at the surface of bare and capped nanoparticles defines the selectivity of the nanocatalyst for alkene in alkyne hydrogenation reaction. Addition of primary alkylamines to platinum and CoPt3 nanoparticle catalysts can drastically increase selectivity for alkene from 0 to more than 90% with about 99.9% conversion. Increasing the primary alkylamine coverage on the nanoparticle surface leads to a decrease in the binding energy of octenes and eventual competition between octene and primary alkylamines for adsorption sites. At sufficiently high coverage with primary alkylamine, the alkylamines dominate, which prevents further hydrogenation of alkenes into alkanes. Primary amines with different lengths of carbon chains have similar adsorption energies at the surface of catalysts and, consequently, the same effect on selectivity. When the adsorption energy of capping ligands at the catalytic surface is lower than adsorption energy of alkenes, the ligands do not affect the selectivity of hydrogenation of alkyne to alkene. On the other hand, capping ligands with adsorption energies at the catalytic surface higher than that of alkyne reduce its activity resulting in low conversion of alkynes.
The significance of this work is that catalysts with improved selectivity can profoundly reduce the amount of generated chemical waste. A clear understanding of the role of capping ligands on chemically synthesized nanoparticles while undergoing catalytic reactions has until now been far from complete. This systematic investigation reveals the role of nanocatalyst surface modification in alkyne hydrogenation.
More information: S.G. Kwon et al., Capping Ligands as Selectivity Switches in Hydrogenation Reactions. Nano Lett.. 12, 5382 (2012).
Journal information: Nano Letters
Provided by Argonne National Laboratory