Discovering the keys to improved biofuel catalysts
(Phys.org)—Scientists at the U.S. Department of Energy's (DOE) Ames Laboratory are learning more about how nano-scale catalytic systems work, and their research could be the key to improved processes for refining biofuels and producing other chemicals.
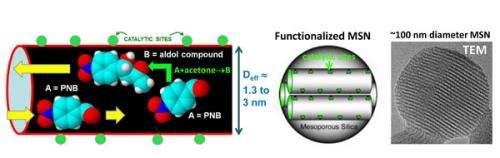
Nanospheres, tiny spheres of silica with a honeycomb of tunnels, or pores, throughout their structure and embedded with catalytic groups, were developed in the last decade as a solution to finding a reusable catalyst for converting biomass into fuel.
While scientists are now able to produce these nanospheres in ways that control the size of the pores and the type and position of the catalytic groups, understanding precisely how these chemical reactions take place will allow further fine-tuning and predictable control of catalytic processes.
A collaborative team of scientists at the laboratory's Division of Chemical and Biological Sciences have determined that though these particles were designed with hollow passages specifically to maximize the surface area available for chemical reactions, these reactions don't happen uniformly across the entire surface area of the particle.
These issues prompted James Evans to develop a new theoretical model which allows better predictions of how these complex systems will behave. Evans is an Ames Laboratory faculty scientist and Professor in Physics and Astronomy at Iowa State University who specializes in theoretical and computational tools for understanding non-equilibrium processes, including catalysis,
He describes the reaction behavior as being similar to a busy grocery store, where customers roam multiple aisles, grabbing items off the shelves. Because the aisles get pretty full of customers throughout, most of the action will occur near the ends of the aisles, where shoppers can get in easily, grab items, and leave easily. Shoppers in the middle of the aisles will have a harder time passing each other and getting out of the aisle with their items. In the same way, the chemical reactions deep within the pores are limited.
"The catchphrase we use to describe restricted passing in narrow pores (or aisles) is single file diffusion. In this situation, reaction is controlled by the random or stochastic nature of molecular motion near the pore openings. So traditional reaction-diffusion equations which do not incorporate these stochastic features fail completely to describe reaction behavior," said Evans.
"Suitably refining traditional equations to incorporate stochastic behavior provides an efficient and reliable model to describe dependence of reaction behavior on key system parameters and guides the experimentalist in thinking about the design of nanoporous materials," said Evans.
Evans and graduate students David M. Ackerman and Jing Wang published their findings in a recent issue of Physical Review Letters.
Igor Slowing, a scientist at the laboratory who synthesizes the particles and performs reaction studies demonstrated a dramatic decrease in catalytic yield with decreasing pore diameter. This is consistent with general expectations of transport limitations in these reaction systems for narrow pores, and with the perception that most of the "action" occurs near pore openings.
"What Jim has done is develop a model to help us understand better what key properties we need to change in the material to get the desired results," said Slowing.
"You can imagine there are several possible remedies for what we are observing," said Marek Pruski, the Ames Laboratory scientist who heads up the research team. Pruski specializes in the nuclear magnetic resonance (NMR) studies of the nano-scale particles. "You can adjust the pore diameter to facilitate passing, or reduce the aspect ratio so that pores are better utilized."
The limitations in reaction efficiency predicted by the model developed by Evans and his co-workers were confirmed in a recent Ames Laboratory study oriented to optimize a reaction routinely used in chemical manufacturing and biofuel production. This study combined new NMR methods with kinetic and surface analyses to detect the formation of catalyst inhibitors that tend to reduce the size of the pores, down to a point they behave similar to a case of single file diffusion. The researchers found that increasing the pore size by less than one nanometer improved the activity more than twenty times. Further modifications to the catalyst allowed the researchers to prevent the formation of inhibitors, which eliminates the bottleneck without making the pore wider.
The study, written by Slowing, Pruski, and a team of scientists from Ames Laboratory and Iowa State University's Chemistry Department, was published in the Journal of Catalysis.
"What really inhibits the performance of these systems is poorly understood or misunderstood, and that's why the research being done here is so fundamentally important," said Pruski. "Ultimately, if we want to improve the catalysts, we need to have a clear understanding of the basic phenomena taking place within the pores.
Provided by Ames Laboratory




















