Researchers identify genetic basis of cardiac, craniofacial birth defects
A group of researchers in Israel, the United States and other nations have made important advances in the rapidly-expanding field of "regenerative medicine," outlining for the first time connections in genetic regulation that normally prevent birth defects in heart and facial muscles.
Some of these problems are surprisingly common – about 1 percent of all people have a congenital heart defect. This basic research will provide a road map to ultimately allow scientists to grow the cell types needed to repair such defects, from stem cells that can be generated from a person's own body.
The findings were published online today in the Proceedings of the National Academy of Sciences.
"Advances in regenerative medicine and developmental biology can now happen because we no longer require human embryos to generate stem cells," said Chrissa Kioussi, a co-author on the study and associate professor in the College of Pharmacy at Oregon State University. "The Nobel Prize this year was awarded to people who discovered how to make stem cells from adult biopsies."
Patient-derived stem cells can in principle be turned into any needed cell type, Kioussi said. The key is understanding the exact regulatory process than tells cells what type they are supposed to turn into, she said, such as a cell on the outside of the left ventricle of the heart.
"Once we understand these genetic controls in sufficient detail, we can not only turn a skin cell into a stem cell, but also turn that stem cell into the type needed for the patient to recover," Kioussi said. "We may eventually be able to grow replacement organs from the patient's cells."
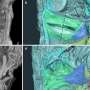
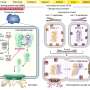
In this study, researchers identified four specific "transcription factor" genes that control processes related to heart and head muscle formation. When there are defects in this process, the result can be death or a range of debilitating problems, from cleft palate to facial malformations and defective heart valves.
"There are about 20,000 genes in the human genome, but only 2,000 of the genes describe transcription factors," Kioussi said. "These transcription factors control the output of genes, the genetic machinery. They collectively determine which of the 20,000 possible molecular machines is actually deployed in each particular cell type."
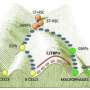
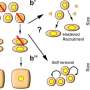
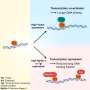
Scientists have found that these transcription factors don't work alone to define cell types in mammalian development – they function in small, self-stabilizing combinations of at least two or three.
The process moves rapidly after conception, and within one month most of the cells in the body "know" their cell type, based on the precise combination of transcription factors produced within them. When researchers understand how these stable transcription factor combinations get generated, they will know how to artificially generate these combinations in stem cells to convert them into the needed cell types.
Mammalian embryonic development is a process of self construction, a series of transitions of "temporary" cell types on the way to adult cell types. A fertilized egg is essentially a stem cell with the potential to become any other cell type. At each intermediate stage, the "temporary" cell types become more restricted in what they can become, until they ultimately achieve and maintain the adult type.
"In this work and in regenerative medicine, we care a great deal about all of these steps of cell differentiation," Kioussi said. "If you know all the steps it takes to get from here to there, you can identify what went wrong and find ways to fix it. This is being done already with some disease problems, and this work will move us closer to being able to repair heart and craniofacial defects."
The task is complex, Kioussi said, but very possible. Although there are 100 trillion cells in the human body, there are only about 100 adult cell types. Understanding and influencing the genetic specification of those cell types is possible and will probably revolutionize the treatment of many defects and diseases, Kioussi said.
This work was supported by the European Research Council, the Israel Science Foundation, the U.S. National Institutes of Health, and other agencies. The lead author was Eldad Tzahor at the Weizman Institute of Science in Israel, and other collaborators were from universities and agencies in the United Kingdom, India and Spain.
Journal information: Proceedings of the National Academy of Sciences
Provided by Oregon State University