Researchers optimize photoluminescent probes to study DNA and more
Sorting good data from bad is critical when analyzing microscopic structures like cells and their contents, according to researchers at Rice University. The trick is to find the right window of time through which to look.
A new paper by the Rice lab of Angel Martí, an assistant professor of chemistry and bioengineering, offers a methodology to optimize the sensitivity of photoluminescent probes using time-resolved spectroscopy. Martí and co-author Kewei Huang, a graduate student in his group, found their technique gave results nearly twice as good as standard fluorescence spectroscopy does when they probed for specific DNA sequences.
Their results were reported recently in the American Chemical Society journal Analytical Chemistry.
In spectroscopy, chemicals and materials from proteins to nanotubes can be identified and tracked by their fluorescence—the light they return when excited by an input of energy, usually from a laser. In the kind of targeted spectroscopy practiced by Martí and his colleagues, a luminescent probe called a molecular beacon is designed to attach to a target like a DNA sequence and then light up.
Improving a probe's ability to detect ever smaller and harder-to-find targets is important to biologists, engineers and chemists who commonly work on the molecular scale to analyze cell structures, track disease or design tiny machines.
One problem, Martí said, has been that even in an experiment lasting a fraction of a second, a spectrometer can return too much information and obscure the data researchers actually want. "In standard fluorescence spectroscopy, you see noise that overlaps with the signal from your probe, the scattering from your solution or cuvettes, plus the noise from the detector," he said. The saving grace, he said, is that not all those signals last the same amount of time.
Time-resolved spectroscopy provides part of the answer, Martí said. Compared with standard spectroscopy, it's like taking a film instead of a snapshot. "We create a kind of movie that allows us to see a specific moment in the process where photoluminescence is occurring. Then we can filter out the shadows that obscure the measurement or spectra we're looking for," he said.
With samples loaded into the spectrometer, researchers yell "Action!" by firing a laser that excites the target. In an edit of the resulting "movie" (which can be done in real time by the spectrometer), they chop off the front and back to narrow the data set to a range that might last only 80-billionths of a second, when the probe signal is strongest and the background signals are absent.
But it's critical to know just the right window of time to look at, Martí said. That's where the Rice methodology removes any uncertainty. They let researchers analyze all the factors, such as the emission intensity and decay of the specific probe with and without the target and the anticipated level of background noise. The experiment can then maximize the signal-to-background noise ratio. The technique works even with probes that are less than optimal, he said.
In combination with a technique called fluorescence lifetime microscopy, the Rice calculations may improve results from other diagnostic tools that gather data over time, such as magnetic resonance imaging machines used by hospitals.
Martí said the equations were the common-sense results of years of working with fluorescent spectroscopy. But, he said, when he looked for materials to help teach his students how to use time-resolved techniques to improve probes' resolution, he found none.
"I thought there must be some publication out there that would describe the tools we use, but there weren't any," he said. "So we've had to write them."
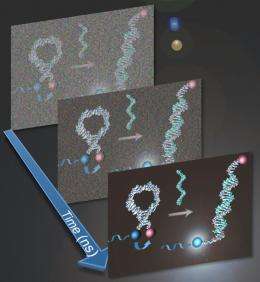
To prove their method, Martí and Huang tested ruthenium- and iridium-based light-switching probes under standard fluorescent and time-resolved spectroscopy. The hairpin-shaped probes' middles are designed to attach to a specific DNA sequence, while the ends are of opposite natures. One carries the fluorophore (iridium or ruthenium), the other a chemical quencher that keeps the fluorescence in check until the probe latches onto the DNA. When that happens, the fluorophore and the quencher are pulled apart and the probe lights up.
The individual signal is a flash too tiny and quick for the naked eye to see. "But our instruments can," Martí said. "We're trying to show that you can use time-resolved spectroscopy for many applications, but to use it in the right way, you have to do some analysis first," he said. "If you do it in the correct way, then it's a very powerful technique."
More information: pubs.acs.org/doi/abs/10.1021/ac3019894
Journal information: Analytical Chemistry
Provided by Rice University