Elusive metal discovered

Carnegie scientists are the first to discover the conditions under which nickel oxide can turn into an electricity-conducting metal. Nickel oxide is one of the first compounds to be studied for its electronic properties, but until now scientists have not been able to induce a metallic state. The compound becomes metallic at enormous pressures of 2.4 million times the atmospheric pressure (240 gigapascals). The finding is published in Physical Review Letters.
"Physicists have predicted for decades that the nickel oxide would transition from an insulator—a compound that does not conduct electricity—to a metal under compression, but their predictions have not previously been confirmed," remarked team leader Viktor Struzhkin of Carnegie's Geophysical Laboratory. "This new discovery has been a goal in physics that ranks as high as achieving metallic hydrogen, but for metal oxides."
The outer shells of atoms contain what are called valence electrons, which play a large role in electrical and chemical behavior. Metals generally have one to three of these valence electrons, while non-metals have between five and seven. Metals are good conductors of electricity because the valence electrons are loosely bound, so the electrons are free to flow through the material.
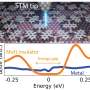
Nickel oxide is what is called a transition metal oxide, which despite its partially filled outer shell of electrons, remains an insulator. The scientists placed thin crystal samples, no more than one millionth of a meter (micron) thick, into a custom-designed diamond anvil cell. Four thin foil leads were crafted to allow the measurements. The researchers were able to measure declining electronic resistance beginning at 1.3 million atmospheres (130 gigapascals). At 2.4 million atmospheres there was a dramatic, three-order-of-magnitude drop in electronic resistance indicating a change from a semiconducting to a metallic state. The metallic part of the material was located in the region of highest compression.
"This finding is certainly important in providing a better understanding of advanced electronic materials," said Alexander Gavriliuk, first author of the publication and a visiting scientist at Carnegie's Geophysical Laboratory. "But it also gets us closer to the ultimate goal of the condensed matter science—improving theory so it can predict the properties of new materials and then guiding their preparation for practical use."
Journal information: Physical Review Letters
Provided by Carnegie Institution for Science