Depleted uranium: could this reduce our dependency on crude oil?
(Phys.org) -- A simple three-step chemical reaction which could herald the introduction of new sustainable feedstocks for the chemical industry has been developed by scientists at The University of Nottingham.
Scientists in the School of Chemistry have developed a recyclable system for converting carbon monoxide (CO) directly into more complicated organic molecules using depleted uranium.
The research, funded by the Royal Society and European Research Council, was led by Dr. Stephen Liddle, an expert in inorganic chemistry. Details of the new procedure — which can return the molecule that performs the transformation back to its start point — have been published in the prestigious academic journal Proceedings of the National Academy of Sciences (PNAS).
Many of the products society has come to rely on are derived from petrochemicals and an array of feedstocks needed to satisfy the voracious appetite of the petrochemical industry are produced from the refining of crude oil.
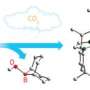
The search for more sustainable sources of feedstocks for petrochemical production is gathering pace and CO is, in principle, one of the options. Industry generates CO in abundance but the problem facing chemists has been how to convert CO directly into useable molecular compounds.
Dr. Liddle said: “This is a significant step forward in the search for viable alternatives to crude oil — it means that a simple catalytic process for converting CO directly into more complex and value-added organic molecules may soon be in reach.”
The need for alternative sources of feedstocks
The continued growth and stability of the global economy requires the ready availability of petrochemical feedstocks. However, uncertainty in their cost and supply and events such as the energy crisis in the 1970s has driven demand for developing alternative sources to crude oil.
Derived from methane and coal stocks, CO is an important industrial gas widely used in many industrial operations — for example in the production of aldehydes, methanol and pure nickel. It is readily produced by steam reforming reactions and as a result is an abundant resource that could be used in crude oil-free bulk hydrocarbon feedstock production.
But developing processes to fix CO into molecules of use are energy intensive. Many attempts have been made to make molecular compounds derived purely from CO fixation but they aren’t recyclable.
What happens next?
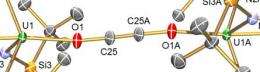
Using depleted uranium, chemists at The University of Nottingham have developed a three stage synthetic cycle in which an electron is transferred to CO molecules making them more reactive, a new molecule is then assembled, and then, crucially, the CO-derived molecule is liberated and the metal complex that creates the chemical reaction is retrieved so it can be used again.
Dr. Liddle said: “Our work represents a step forward because we have closed a simple synthetic cycle for fixing carbon monoxide — the challenge now is to make this a catalytic cycle and to exploit the findings of this work with metals that industry could more easily use.”
Journal information: Proceedings of the National Academy of Sciences
Provided by University of Nottingham