Bubbles help break energy storage record for lithium-air batteries
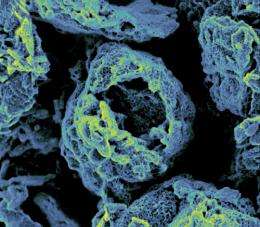
Resembling broken eggshells, graphene structures built around bubbles produced a lithium-air battery with the highest energy capacity to date, according to scientists at Pacific Northwest National Laboratory and Princeton University. This black, porous material could replace the traditional smooth graphene sheets in lithium-air batteries, which become clogged with tiny particles during use. As an added bonus, the team’s new material does not rely on platinum or other precious metals, reducing its potential cost and environmental impact.
"This hierarchical structure of self-assembled graphene sheets is an ideal design not only for lithium-air batteries but also for many other potential energy applications," said Dr. Jie Xiao, the materials scientist at PNNL who led the study.
Lithium-air batteries could allow for the creation of long-range electric vehicles, able to travel up to 300 miles between charges. Comparatively lightweight, lithium-air batteries still suffer from limited practical capacity and poor cycle life issues. However, this study showed how to maximize the capacity of the batteries.
"This is critical for applications, including electric vehicles and energy storage," said Dr. Jun Liu, a materials scientist on the study and Director of PNNL’s Transformational Materials Science Initiative, which funded the research.
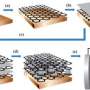
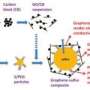
The team began by combining a binding agent with graphene, a special form of carbon. The binding agent dispersed the graphene in solution, like soap disperses grease in dishwater. The graphene and binder were then added to water and mixed using a process that created bubbles inside the solution. The graphene and binder formed and hardened around the bubbles. When the bubbles eventually popped, hollow spheres of graphene were left behind. The tiny black particles are only 3 to 4 microns in diameter, ten times smaller than a human hair.
Using both modeling and microscopy, the scientists analyzed the graphene structures and their performance. They performed density functional theory calculations on the supercomputing system at the National Energy Research Scientific Computing Center. They studied the particles using electron microscopy at the Environmental Molecular Sciences Laboratory.
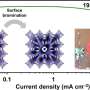
The researchers found that the black porous structures store more than 15,000 milliamp hours per gram of graphene, making it far denser in term of energy capacity than other materials.
"Many catalysts are studied now for this technology. In our process we chose not to use precious metal," said Dr. Ji-Guang Zhang, the group leader in PNNL's Li-air battery research. "This will greatly reduce production costs and increase the adoptability."
The battery is achieving the highest levels of energy capacity in an oxygen-only environment. When operated in ambient air, the capacity drops because the water in the air fouls the lithium metal in the batteries. The PNNL team is working to develop a membrane to block the water and still allow the necessary oxygen to flow
"We also want to make the battery rechargeable," said Zhang. "Right now, it is not. It is not fully rechargeable. We are working on a new electrolyte and a new catalyst so that the battery can be recharged multiple times, potentially for battery backup applications that require high energy densities."
More information: J Xiao, D Mei, X Li, W Xu, D Wang, GL Graff, WD Bennett, Z Nie, LV Sara, IA Aksay, J Liu, and JG Zhang. 2011. "Hierarchically Porous Graphene as a Lithium-Air Battery Electrode." Nano Letters. DOI: 10.1021/nl203332e
Journal information: Nano Letters
Provided by Pacific Northwest National Laboratory