This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers discover an effective and environment-friendly disinfectant
A widely used disinfectant worldwide, chloroxylenol, has been associated with eco-toxicological threats in water environments due to its relatively high chemical stability and massive consumption. Researchers at the School of Engineering of the Hong Kong University of Science and Technology (HKUST) have discovered a promising alternative known as 2,6-dichlorobenzoquinone (2,6-DCQ), which works more effectively in combating certain common bacteria, fungi and viruses, and can be rapidly degraded and detoxified in receiving waters.
Their findings have been published in Nature Communications.
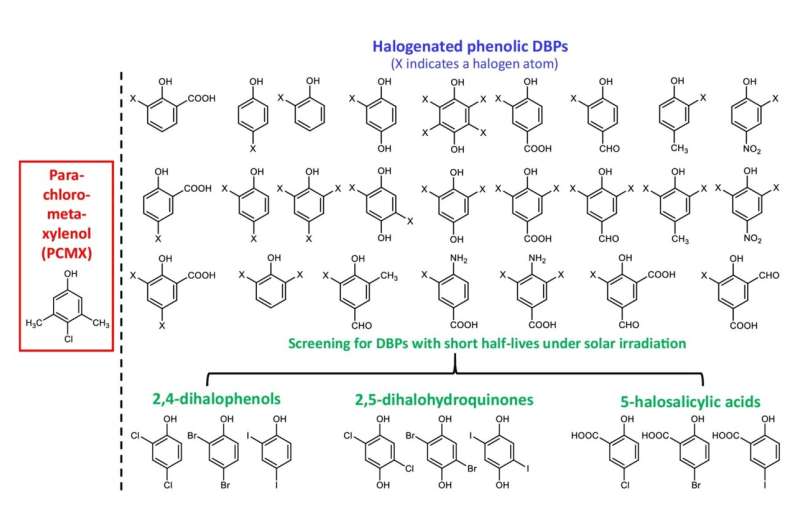
This study was led by Prof. Zhang Xiangru from HKUST's Department of Civil and Environmental Engineering, who has been studying disinfection byproducts (DBPs) for many years. During the pandemic outbreak, Prof. Zhang noticed that chloroxylenol is structurally similar to some halo-phenolic DBPs previously discovered by his team, which have been shown to rapidly degrade by solar photolysis.
Inspired by the structural property and degradability of some halo-phenolic DBPs, the research team managed to select an effective broad-spectrum disinfectant from the DBPs that can be rapidly degraded and detoxified in receiving waters.
The research team tested the efficacy of 10 different DBPs in inactivating various pathogens, including E. coli (a type of bacteria associated with colorectal cancer), Staphylococcus aureus (bacteria), Candida albicans (fungi), and bacteriophage MS2 (viruses). They found that 2,6-DCQ was nine to 22 times more effective than chloroxylenol in inactivating these bacteria, fungi, and viruses.
Furthermore, they found that the developmental toxicity of 2,6-DCQ to marine polychaete embryos decreased quickly due to its rapid degradation via hydrolysis in receiving seawater, even in the absence of sunlight. Two days after being discharged into seawater, 2,6-DCQ exhibited 31 times lower developmental toxicity compared to chloroxylenol.
"We discovered that the selected DBP exhibited substantially stronger antimicrobial efficacy than chloroxylenol and that its concentration and associated developmental toxicity in receiving seawater decreased rapidly, even in darkness," Prof. Zhang said.

He emphasized the pressing need for more effective and eco-friendly disinfectants, particularly in the wake of the COVID-19 pandemic.
"Chloroxylenol has been frequently detected in aquatic environments; for instance, its concentration has reached up to 10.6 μg/L in river water in Hong Kong. Toxicological studies have reported adverse effects of chloroxylenol on aquatic organisms, including endocrine disruption, embryonic mortality, and malformations. Chronic exposure to chloroxylenol at environmental concentrations (~4.2 μg/L) can cause gene regulation and morphological changes in rainbow trout."
The team's discovery of 2,6-DCQ as a promising alternative is an important step towards addressing this global need. The results suggest that 2,6-DCQ may be used as a disinfectant on a wide range of occasions, including personal care products (such as hand cleansers, detergent, and soap), paint, textiles, metal working fluids, medical scrubs, as well as sanitation for households, food processing equipment, surgical instruments, and public places.
"This innovative study not only provides a potential solution to better support human biosecurity while prioritizing environmental sustainability, but also carries significant implications for the development of green disinfectants and other green industrial products by exploiting the slightly alkaline nature of seawater. For example, scientists may design and develop other industrial products such as pesticides, pharmaceuticals, and personal care products that can be rapidly degraded by hydrolysis in receiving seawater," Prof. Zhang said.
The research team included Dr. Han Jiarui, currently a Research Assistant Professor at HKUST, and Dr. Li Wanxin, currently an Assistant Professor at Xi'an Jiaotong-Liverpool University. They are both Ph.D. graduates from HKUST's Department of Civil and Environmental Engineering and were postdoctoral fellows in Prof. Zhang's group during the study.
Looking ahead, Prof. Zhang plans to explore the relationships between disinfection efficiency and degradability of halophenols with their molecular fingerprints through machine learning. He hopes future investigations will shed light on the further development of optimal disinfectants.
More information: Jiarui Han et al, An effective and rapidly degradable disinfectant from disinfection byproducts, Nature Communications (2024). DOI: 10.1038/s41467-024-48752-w
Journal information: Nature Communications
Provided by Hong Kong University of Science and Technology