Exploring how adding UV treatment to water chlorination can actually increase toxic trihalomethane production
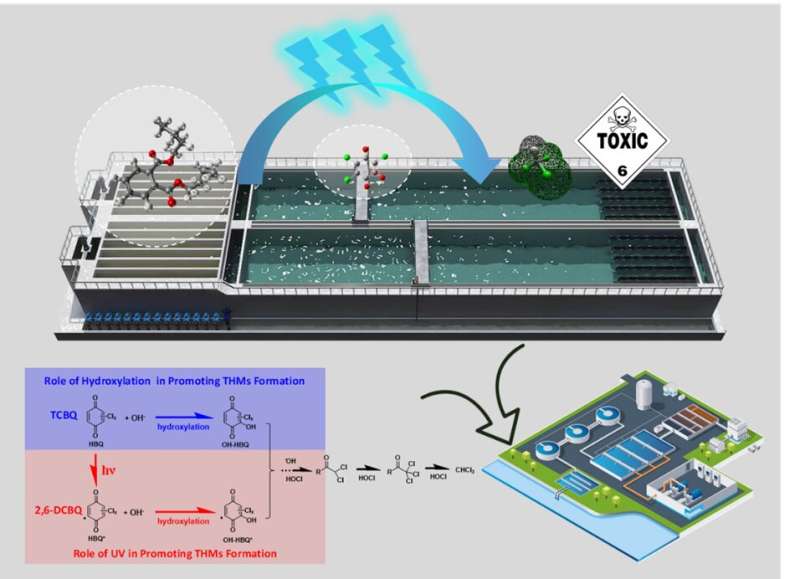
Halobenzoquinones (HBQs), as new emerging disinfection by-products (DBPs), are frequently detected in potable and swimming pool waters. In fact, HBQs are also precursors of other DBPs such as currently regulated trihalomethanes (THMs), which pose a high risk to the public health and the environment. When UV is applied during the chlorination process, the DBPs formation may be quite different from that under chlorine alone or UV alone. However, there remain many questions that require further investigation. For instance, does UV/chlorine combined disinfection promote or limit the transformation of HBQs into DBPs compared with chlorine-only disinfection? Particularly, does UV play a significant role in the transformation of organic precursors and the formation of DBPs upon chlorine disinfection?
To answer these questions, Prof. Ching-Hua Huang from the Georgia Institute of Technology, Dr. He Zhao from the Institute of Process Engineering Chinese Academy of Sciences and their team members have worked jointly and revealed systematically the molecular mechanism of THMs produced from HBQs in chlorination or combined UV/chlorine. Their work identified the promoting effect of UV on HBQs in THMs formation, which provided valuable insight for the potential risk during the application of combined UV/chlorine in water treatment. The study was published in Frontiers of Environmental Science & Engineering.
In this study, the research team found UV enhanced by nearly 10 times the CHCl3 formation yield of 2,6-dichloro-1,4-benzoquinone (2,6-DCBQ, one of the most frequently detected HBQs in drinking water). They also conducted in-depth investigation of the reaction mechanism using complementary experimental measurements and density functional theory (DFT) calculations, and revealed different formation patterns of THMs from four species of HBQs during chlorine-only and UV/chlorine processes. Their investigation showed that UV had a great influence according to the chemical structure of HBQs, directly affecting the hydrolysis rate, transformation pathways, intermediates, and the subsequent production of THMs. Their results also unveiled the hydroxylation of HBQ to be a key intermediate pathway in promoting THM formation.
This study investigated comprehensively and systematically the formation of THMs from HBQs during chlorination or combined UV/chlorine processes for the first time, which should be considered in the application of combined UV and chlorine processes in water treatment. It has deepened our understanding of the promoting roles of UV irradiation and hydroxylation of HBQs in THMs formation, which provides a new insight for the potential risks for UV disinfection of drinking water treatment.
More information: He Zhao et al, Enhanced formation of trihalomethane disinfection byproducts from halobenzoquinones under combined UV/chlorine conditions, Frontiers of Environmental Science & Engineering (2021). DOI: 10.1007/s11783-021-1510-7
Provided by Higher Education Press