This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Scientists take atomic look at a protein complex that grants access to our DNA
To transcribe the information contained in our genes or to repair the dozens of breaks that occur daily in our DNA, our enzymes must be able to directly access the DNA to perform their functions. However, in the cell nucleus, this access is limited because the DNA strands are often tightly coiled and packed around proteins like threads around spools.
Researchers from Lawrence Berkeley National Laboratory (Berkeley Lab), UC Berkeley, the Institute for Systems Biology, and Université Laval now have a better understanding of the protein complex that creates access to packed DNA, TIP60.
Knowing the detailed structure and behavior of TIP60 could provide insight into different diseases where the protein complex plays a role, such as Alzheimer's and various cancers. The work was reported in the journal Science on August 1.
"This collaborative work brings together structure and functional assays in a powerful way to inform us on how this complex macromolecular assembly carries out its job to regulate the reading of our genome," said Eva Nogales, a senior faculty scientist at Berkeley Lab, UC Berkeley professor, and Howard Hughes Medical Institute investigator.
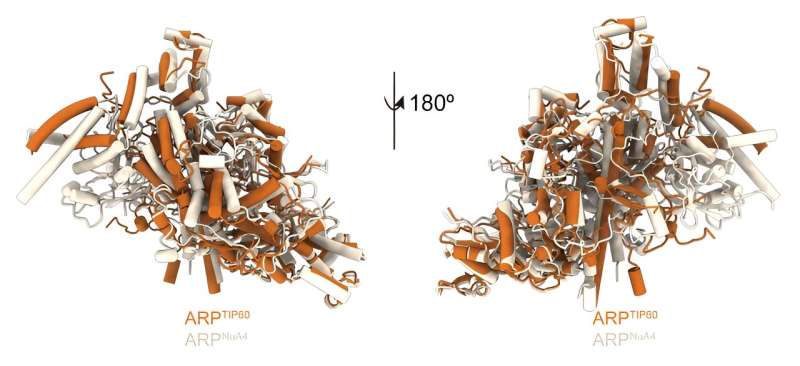
"The structure of the human TIP60 reveals how evolution has led to the merging of two distinct molecular functions into a single complex, readjusting the way structural modules come together to fit its dual functionality."
The researchers were able to study the structure of this complex, which is made up of 17 proteins, and the interactions between its components. They used several approaches, including high-resolution cryo-electron microscopy at the Nogales lab at UC Berkeley. This technology, which earned three scientists the Nobel Prize in Chemistry in 2017, allows scientists to see the structure of proteins at the atomic scale.
"High-resolution cryo-electron microscopy makes it possible to study the molecular structure of complex biological systems such as proteins, something that no other method has previously allowed," explained Jacques Côté, professor at the Faculty of Medicine at Université Laval, researcher at the CHU de Québec-Université Laval Research Center, and co-leader of the study.
To shed light on the structure of TIP60, Nogales and her team at Berkeley Lab and UC Berkeley purified and studied samples prepared by the Côté group. "Professor Nogales not only has access to the specialized equipment required to perform this type of analysis, but her expertise in high-resolution cryo-electron microscopy is recognized worldwide," he said.
TIP60 malfunction is associated with several types of cancer, including colon, lung, breast, pancreatic, stomach, and metastatic melanoma. It is also associated with neurological disorders such as Alzheimer's.
"When access to DNA is restricted, the enzymes that repair DNA breaks cannot function, and significant cellular damage can occur," Côté said. "The same problem can occur with tumor suppressor genes. For them to be expressed, TIP60 must be able to create an opening into the DNA."
Côté said that a good understanding of the structure of TIP60 is essential if we hope to develop new targeted therapies for diseases associated with low levels of TIP60, including Alzheimer's.
"For these diseases, we could develop molecules that bind to the active sites of TIP60 in order to activate it," Côté said.
He added that for cancers, administering TIP60 inhibitors to affected tissues could perhaps locally slow the multiplication of cancer cells.
"For the moment, there are no good TIP60 inhibitors," he said. "Now that the structure of this complex is known, we hope that things will get moving."
More information: Zhenlin Yang et al, Structural insights into the human NuA4/TIP60 acetyltransferase and chromatin remodeling complex, Science (2024). DOI: 10.1126/science.adl5816
Journal information: Science
Provided by Lawrence Berkeley National Laboratory