3D structure of artificially designed protein nanoparticle TIP60 elucidated by cryo-electron microscopy
Nanoparticles and nanocages are attractive materials that may be applied in color agents, catalysts, and drug delivery. For real-world use, it is necessary to produce a large number of nanoparticles of uniform size and shape, but thus far, nanoparticle formation methods using metals have been widely researched, and the formation of nanoparticles with a certain shape and size have been realized. However, it is not easy to create a group of uniform nanoparticles with the same structure at the atomic level.
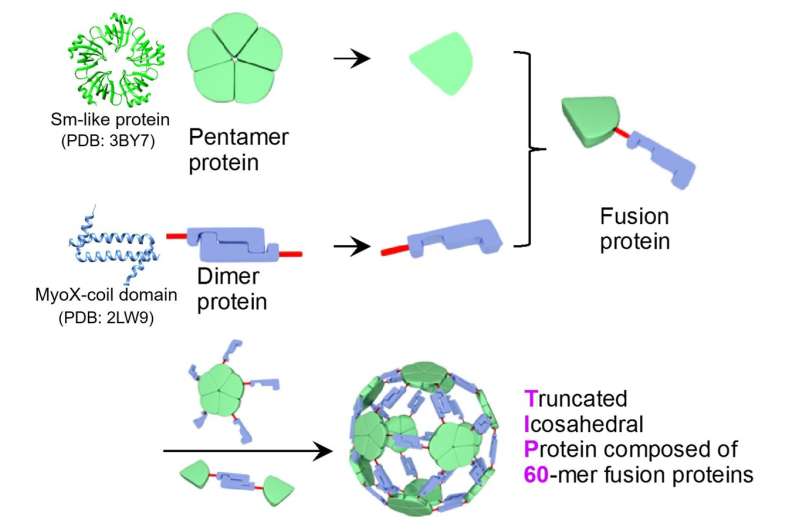
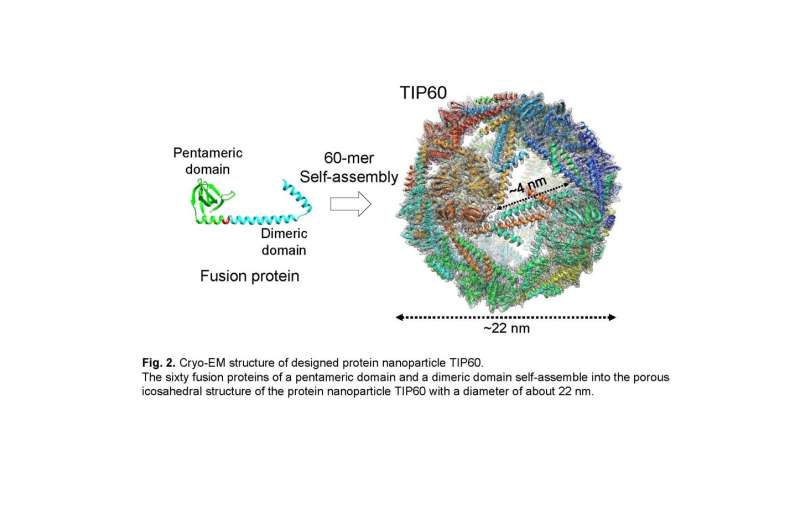
A joint research group led by Associate Professor Ryoichi Arai (Institute for Biomedical Sciences and Faculty of Textile Science and Technology, Shinshu University) and Assistant Professor Norifumi Kawakami (Faculty of Science and Technology, Keio University) developed a uniform and useful supramolecular protein nanoparticle symmetrically self-assembled from fusion proteins of a pentameric protein domain and a dimeric protein domain. It is possible to modify the functionality by site-specific mutagenesis or chemical modification. This designed protein nanoparticle with a diameter of about 22 nm was named TIP60 (Truncated Icosahedral Protein composed of 60-mer fusion proteins) because it is formed by self-assembling 60-meric artificial fusion proteins shaped like a soccer ball.
In the present study, the joint research group solved the detailed three-dimensional structure of the TIP60 using single-particle cryo-electron microscopy. A large amount of TIP60 was expressed in E. coli, and a purified sample was observed at the cryo-electron microscope facility operated by Prof. Masahide Kikkawa lab at the University of Tokyo. By performing single-particle analysis based on obtained image data, a three-dimensional map was reconstructed with a resolution of 3.3 Å. It was revealed that TIP60 forms hollow spherical nanoparticles as designed and has an icosahedral 60-meric structure with 20 triangular-like pores with an edge of about 4 nm each. In addition, the group elucidated in detail the characteristic three-dimensional structure, such as the linker connecting the pentamer formation domain and the dimer formation domain composed of an α-helix.
When a small molecule compound is added after chemically modifying only the outer surface of TIP60 with a high molecular compound, the small molecule compound enters the internal cavity and chemically modifies in the inner surface. In other words, it was found that the porous structure of TIP60 acts as a filter by molecular size, and the outer and inner surfaces of TIP60 can be chemically modified with different molecules of different sizes.
In the future, the group will utilize artificially designed protein nanoparticles by advancing the design and functional modification of site-specific variants based on the three-dimensional structure of TIP60 elucidated in this study. It is expected to lead to the development and applications in the nanobiotechnology and nanomaterial fields, such as use as a nanocapsule for a drug delivery system.
The research was published in Chemical Communications.
More information: Junya Obata et al, Icosahedral 60-meric porous structure of designed supramolecular protein nanoparticle TIP60, Chemical Communications (2021). DOI: 10.1039/D1CC03114G
Journal information: Chemical Communications
Provided by Shinshu University