This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
Researchers create ADP- or ATP-containing molecules with improved yield and consistency
Adenosine diphosphate (ADP) or adenosine triphosphate (ATP)-containing important biological molecules can modify macromolecules like proteins and nucleic acids to alter their function in the cell. Synthesizing ADP- and ATP-containing molecules using traditional methods is challenging, however.
A new chemical reaction has greatly improved the amount of synthesized ADP- and ATP-containing molecules created during a reaction, allowing researchers to use and better understand the function of these molecules in the cell.
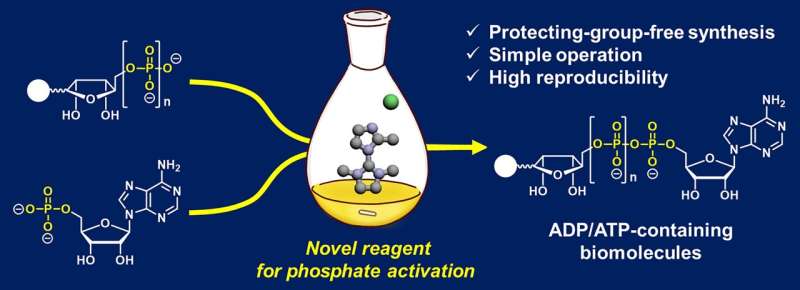
Traditional methods of generating ADP- and ATP-containing molecules suffer many drawbacks, including complex procedures, drying conditions, low reproducibility and low reaction yields. Additionally, many of these reactions can only accommodate a narrow range of molecules that can construct an ADP or ATP framework, limiting their usefulness.
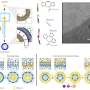
To address this issue, a team of researchers developed a new chemical reaction that reliably produces ADP- and ATP-containing molecules with high yields. To do this, the team modified a coupling reaction to create ADP- and ATP-containing molecules including nicotinamide adenine dinucleotide (NAD+, a molecule involved in energy metabolism) analogs.
The team published the study in the July 19 issue of Chemistry: A European Journal.
"The problem with traditional coupling reactions is the lack of reproducibility in chemical synthesis of ADP- and ATP-containing molecules, which are necessary for molecular-level studies to elucidate their detailed biological functions via conventional methods. This lack of reproducibility is due to the water content in highly polar phosphate-containing substrates," said Hide-Nori Tanaka, associate professor in the Institute for Glyco-core Research (iGCORE) and The United Graduate School of Agricultural Science at Gifu University in Gifu, Japan and senior author of the paper.
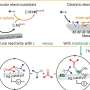
Traditional methods of constructing an ADP or ATP framework often require phosphate-protecting groups that mask the chemistry of functional groups on a molecule that could interfere with a reaction. In this case, protecting groups were necessary to shield the phosphate groups, which are negatively charged, from interacting with water. These protecting groups subsequently need to be removed once the coupling reaction has occurred.
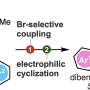
Alternatively, other reactions use 2-(N-imidazoyl)-1,3-dimethylimida-zolinium chloride (ImIm-Cl) to create a pyrophosphate bond (loss of a water molecule that occurs when two phosphates bind) between one phosphate and another phosphate without the need for protecting groups. These reactions limit the molecules that can be linked to ADP, however, and do not produce high yields.
"To address [the synthesis] problem, we developed an efficient and reliable method enabling reproducible and high-[yield] access to ADP- and ATP-containing molecules through [a] protecting-group-free reaction using a hydrolysis-stable reagent for phosphate activation," said Tanaka.
Specifically, the research team created a more efficient coupling reaction by modifying ImIm-Cl to make the molecule stable in water, eliminating the water content issue. To achieve this, the researchers introduced a small methyl group (-CH3) to ImIm-Cl to make an ImIm-Cl analog, 2-MeImIm-Cl, that was hydrophobic, or hardly hydrolyzed in water.
Remarkably, optimized coupling reactions using 2-MeImIm-Cl achieved high yields with a variety of different molecules. ADP-ribose (ribose is a 5-carbon sugar) derivatives achieved yields between 55 and 75%. NAD+ analogs reached yields of 53 to 84%. Challenging ATP formations ranged between 46 and 67% yields. ADP-ribosyl peptides, representing a larger molecular coupling, were also generated at 65 to 70% yields.
The new reaction improves the efficiency, yield and complexity of traditional coupling methods, and provides researchers with a more practical method of synthesizing ADP- and ATP-containing molecules.
"The next step is to synthesize structurally diverse and defined ADP- and ATP-containing biomolecules, such as ADP-ribosyl peptides/proteins, nucleoside oligophosphates and oligo/poly ADP-ribose, using this method. Our ultimate goal is to elucidate their detailed functions [with a] chemical biology approach using synthetic molecules," said Tanaka.
Rui Hagino from The United Graduate School of Agricultural Science at Gifu University in Gifu, Japan; Ryo Kuwabara from the Department of Applied Bioorganic Chemistry at Gifu University; Naoko Komura from the Institute for Glyco-core Research (iGCORE) at Gifu University, Akihiro Imamura and Hideharu Ishida from iGCORE, The United Graduate School of Agricultural Science, and the Department of Applied Bioorganic Chemistry at Gifu University; and Hiromune Ando from iGCORE and The United Graduate School of Agricultural Science at Gifu University also contributed to this research.
More information: Rui Hagino et al, Protecting‐Group‐Free Synthesis of ADP‐Ribose and Dinucleoside Di‐/Triphosphate Derivatives via P(V)‐P(V) Coupling Reaction, Chemistry – A European Journal (2024). DOI: 10.1002/chem.202401302
Journal information: Chemistry – A European Journal
Provided by Tokai National Higher Education and Research System