This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers reveal novel mechanism of enhanced P450 demethylase activity through engineered key gating residues
A crucial step in the degradation and utilization of lignin is the process of O-demethylation of lignin monomers, facilitated by O-demethylases. Current O-demethylases face challenges such as limited substrate specificity, unclear reaction mechanisms, and a lack of reductase chaperones. Therefore, the exploration of new O-demethylases through gene mining and the improvement of existing O-demethylases through protein engineering are of great importance.
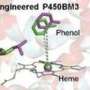
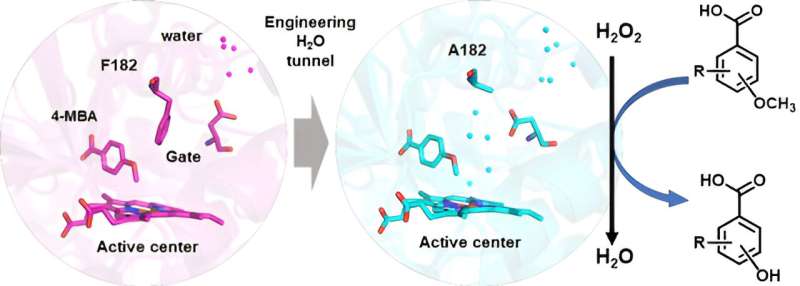
The cytochrome CYP199A4 monooxygenase from Rhodopseudomonas palustris strain HaA2 is one of the few cytochrome P450 enzymes known to catalyze the demethylation of lignin monomer derivatives. However, its demethylation activity depends on the presence of the nicotinamide cofactor NADH and a reducing chaperone protein.
A team of researchers led by Prof. Cong Zhiqi from the Qingdao Institute of Bioenergy and Bioprocess Technology of the Chinese Academy of Sciences has transformed the NADH-dependent CYP199A4 into a peroxygenase that can directly utilize H2O2 through an innovative engineering strategy known as H2O2 tunneling.
The results were published in Chemical Science on May 3.
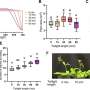
They also developed a library of mutant CYP199A4 peroxygenases to study the O-demethylation of 21 lignin monomer analogs. The engineered CYP199A4 peroxygenase showed a preference for catalyzing the reaction at specific sites, with impressive conversion rates that exceeded or matched those of the natural substrate 4-methoxybenzoic acid.
The researchers also solved the crystal structure of the advantageous mutant at high resolution. Structural analysis revealed that the mutated enzyme, with reduced steric hindrance and specific hydrophobic residues, exhibited an increased presence of water molecules within its active site.
This suggests that H2O2 molecules may have easier access to the active site, supporting a mechanism by which specific amino acid residues modulate the involvement of H2O2 in the reaction.
In addition, the researchers used classical molecular dynamics simulations and advanced sampling techniques to calculate energy barriers for H2O2 passage through the tunnel.
The F182A mutant showed a significantly decreased tunnel potential barrier compared to the wild type, resulting in improved transport of H2O/H2O2 molecules to the heme active site. These results provide a theoretical explanation for the effectiveness of the H2O2 tunneling engineering strategy.
In conclusion, this study not only provides new insights into the development of synthetic P450 peroxygenases for the organic transformation of valuable compounds, but also highlights the potential of generating P450 peroxygenases through H2O2 tunneling engineering. This innovation has the potential to expand the applications of P450 enzymes in synthetic chemistry and biology.
More information: Panxia Zhao et al, Crucial gating residues govern the enhancement of peroxygenase activity in an engineered cytochrome P450 O-demethylase, Chemical Science (2024). DOI: 10.1039/D4SC02418D
Journal information: Chemical Science
Provided by Chinese Academy of Sciences