This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Engineering cytochrome P450BM3 enzymes enables direct nitration of unsaturated hydrocarbons

Biocatalytic nitration is a promising method for the preparation of nitro compounds. However, few nitration reactions have been developed using biocatalysis due to the lack of native nitrating enzymes and/or the narrow substrate scope of the enzymes.
Recently, a research team led by Prof. Cong Zhiqi from the Qingdao Institute of Bioenergy and Bioprocess Technology (QIBEBT) of the Chinese Academy of Sciences (CAS) has developed new engineering Cytochrome P450BM3 enzymes for direct nitration of unsaturated hydrocarbons.
The study was published in Angewandte Chemie International Edition on Jan. 20.
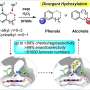
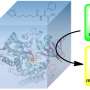
The researchers adopted site-directed mutations of key residues at the active sites to regulate steric effects and limit substrate access, which drastically reduced the monooxygenation activity of P450BM3 and increased the peroxidase activity.
On this basis, they realized direct nitration of unsaturated aromatic and terminal aryl olefin compounds. Several phenol and aniline compounds were nitrated to generate ortho- and para-nitration products with moderate to high total turnover numbers. "This represents the first example of direct aromatic nitration by P450 variants using nitrite as a nitrating agent," said Prof. Cong, corresponding author of the study.
Furthermore, they demonstrated that the DFSM-facilitated P450 peroxidase system could be used for nitration of the vinyl group of styrene and derivatives. The mechanism of direct nitrification was further revealed through crystallographic studies and free radical control experiments.
"Our study not only opens a new avenue for biocatalytic nitration, but also provides a new idea for promoting the peroxidase function of P450 enzyme in the field of synthetic chemistry and synthetic biology," said Prof. Cong.
More information: Xiling Wang et al, Engineering Cytochrome P450BM3 Enzymes for Direct Nitration of Unsaturated Hydrocarbons, Angewandte Chemie International Edition (2023). DOI: 10.1002/anie.202217678
Journal information: Angewandte Chemie International Edition
Provided by Chinese Academy of Sciences