This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New approach enables regiodivergent and enantioselective hydroxylation of C-H bonds
Direct selective hydroxylation, one of the most convenient and economical routes employed in C-H oxyfunctionalization, is widely utilized in various of fields including medicine, chemical industry and materials science.
However, it is still challenging to optionally access diverse hydroxylation products from a given substrate bearing multiple reaction sites of sp3 and sp2 C-H bonds.
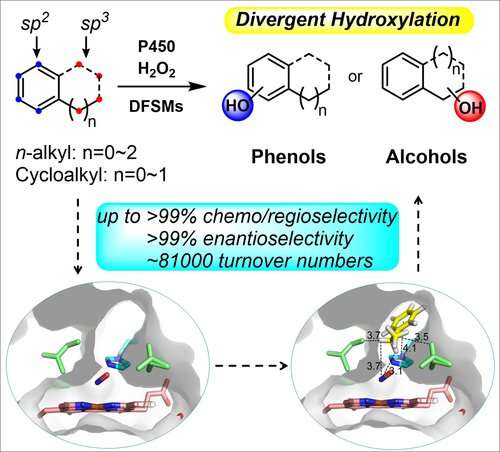
Recently, a research team led by Prof. Cong Zhiqi from the Qingdao Institute of Bioenergy and Bioprocess Technology (QIBEBT) of the Chinese Academy of Sciences (CAS) has developed a new synergistic approach combining protein engineering and exogenous molecules to achieve multi-site highly regio- and enantioselective hydroxylation of alkylbenzenes.
The study was published in Angewandte Chemie International Edition on Nov. 23.
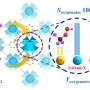
The researchers obtained various combinations of P450BM3 mutants and dual-functional small molecule (DFSM). They used these specific combinations to access more than half of the possible hydroxylated products from each substrate with excellent regioselectivity and enantioselectivity, as well as high total turnover numbers, which is far better than any natural or engineered P450 monooxygenases previously reported.
"The synergistic catalysis induced by the bound DFSMs was key to generating peroxygenase activity, product diversity, and selectivity," said Prof. Cong, corresponding author of the study.
Additionally, the results of crystal structure analysis, molecular dynamic simulations and theoretical calculations provided direct evidence of the synergistic effects between the engineered P450 variants and DFSMs during alkylbenzene hydroxylation, supporting the occurrence of accurately controlled regio- and enantioselectivity at various sites.
"These findings not only demonstrate the power of synergistic use of engineered P450 enzymes and DFSMs to achieve regiodivergent and enantioselective hydroxylation of alkylbenzenes, but also highlight the potential of exogenous molecules in modulating enzymatic catalysis for biochemical transformations," said Prof. Cong.
More information: Jie Chen et al, Regiodivergent and Enantioselective Hydroxylation of C−H bonds by Synergistic Use of Protein Engineering and Exogenous Dual‐Functional Small Molecules, Angewandte Chemie International Edition (2022). DOI: 10.1002/anie.202215088
Journal information: Angewandte Chemie International Edition
Provided by Chinese Academy of Sciences