This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New method links graphene nanolayers for tougher, elastic films
Layers of carbon atoms in a honeycomb array are a true supermaterial: their unusually high conductivity and favorable mechanical properties could further the development of bendable electronics, new batteries, and innovative composite materials for aeronautics and space flight. However, the development of elastic and tough films remains a challenge.
In the journal Angewandte Chemie, a research team has now introduced a method to overcome this hurdle: they linked graphene nanolayers via "extendable" bridging structures.
The special capabilities of microscopic graphene nanolayers often drop off when the layers are assembled into foils, because they are only held together by relatively weak interactions—primarily hydrogen bonds.
Approaches that attempt to improve the mechanical properties of graphene foils by introducing stronger interactions have only been partially successful, leaving particular room for improvement in the stretchability and toughness of the materials.
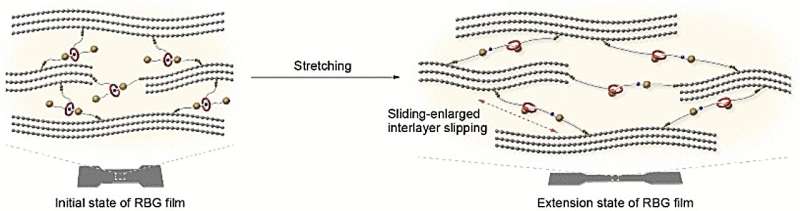
A team led by Xuzhou Yan at Shanghai Jiao Tong University (China) took a new approach: they cross-linked graphene nanolayers with mechanically interlocked molecules whose building blocks are not chemically linked, but rather inseparably spatially entangled. The researchers chose to use rotaxanes as their links.
A rotaxane is a "wheel" (a large ring-shaped molecule) that is "threaded" onto an "axle" (a molecular chain). Bulky groups cap the axles to prevent the wheels from coming unthreaded. The team built their axle with a charged group (ammonium) that holds the wheel in a specific position.
A molecular "anchor" (OH group) was attached to both the axle and wheel by a linker. The graphene was oxidized to make graphene oxide, which forms a variety of oxygen-containing groups on both sides of the graphene layer. These include carboxyl groups, which can bind to the OH groups (esterification). This reaction allows the wheel and axle to cross-link the layers, after which the graphene oxide is reduced back to graphene.
When these films are stretched or bent, the attractive forces between the wheel and the ammonium group on the axle must be overcome, which increases the tensile strength. Increased stress eventually causes the axle to be pulled through the wheel until it "strikes" the end cap. This motion lengthens the rotaxane-bridges, allowing the layers to slide across each other, which significantly increases the stretchability of the film.
Flexible electrodes made from this graphene-rotaxane foil could be stretched up to 20% or bent repeatedly without being damaged. They also retained their high electric conductivity. Only stretching by over 23% led to fracture.
The new foils were considerably stronger than foils without rotaxanes (247.3 vs. 74.8 MPa), as well as more elastic (23.6 vs. 10.2%), and tougher (23.9 vs. 4.0 MJ/m3). The team also built a simple "grasping tool" with mechanical joints that were equipped with and actuated by the new foils.
More information: Chunyu Wang et al, A Stretchable and Tough Graphene Film Enabled by Mechanical Bond, Angewandte Chemie International Edition (2024). DOI: 10.1002/anie.202404481
Journal information: Angewandte Chemie International Edition , Angewandte Chemie
Provided by Wiley