This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
New catalyst brings commercial high-efficiency zinc-air batteries closer to reality
The effective conversion from fossil fuel-based to renewable energy sources requires cost-efficient, high-capacity, rechargeable batteries. Zinc-air batteries (ZAB) can theoretically store large amounts of energy, but current technologies require the use of expensive noble metal catalysts, or agents that speed a chemical reaction, that underperform in charging and discharging reactions.
A new metal-nitrogen-carbon catalyst has been developed for use in ZABs that outperform noble metal catalysts, improving the efficiency and practicality of ZAB technology. ZABs function by oxidizing zinc with oxygen from the air. Recent research demonstrated that a catalyst incorporating a combination of different non-noble metal atoms could increase the rate of discharging reactions and battery performance.
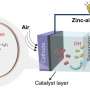
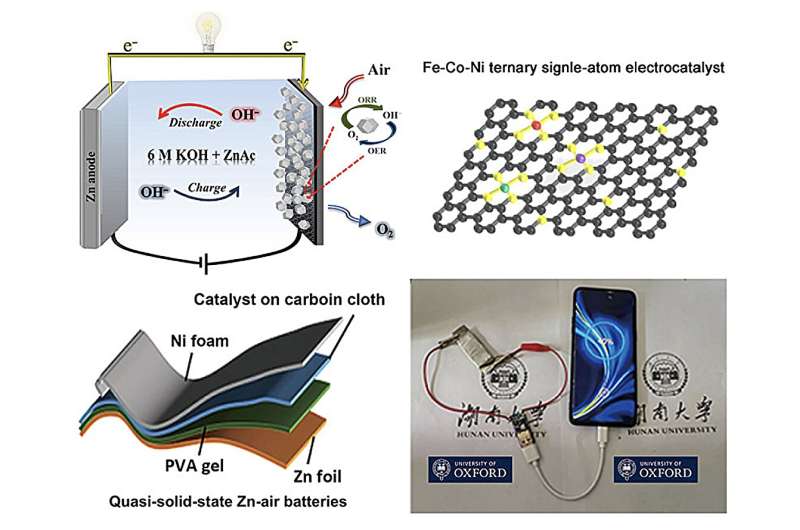
With this evidence in mind, a group of researchers from Hunan University, University College London and the University of Oxford generated a non-noble metal-nitrogen-carbon catalyst from iron, cobalt and nickel (Fe, Co and Ni, respectively) to improve the charging, discharging and cost efficiency of ZABs. Importantly, the team also optimized a flexible carbon dot/polyvinyl alcohol (CD/PVA) film as a solid-state ZAB electrolyte, or battery component that transfers charged atoms, creating a flexible and stable high-performance battery that could potentially be used in wearable devices.
The team published their study in the journal Nano Research Energy on May 17, 2024 .
"Rechargeable metal–air batteries are promising power sources, especially zinc-air batteries (ZABs) which offer high theoretical energy densities (1084 Wh kg−1), environmental friendliness, and cost effectiveness. Additionally, rechargeable ZABs are not only safe and stable but also portable and wearable. Significant research is currently focused on rechargeable and flexible ZABs," said Huanxin Li, research fellow in the Department of Chemistry at the University of Oxford, senior author of the paper and leader of this project.
ZABs discharge and charge through two reactions: the oxygen reduction reaction (ORR) and the oxygen evolution reaction (OER), respectively. These reactions are notoriously slow and require catalysts that speed the electrochemical reaction along, or electrocatalysts. While noble metals are capable of speeding the ORR and OER, issues with cost, suboptimal performance and the requirement of two different noble metals limited the overall practicality of ZAB technology.
"Developing low-cost and efficient bifunctional non-noble electrocatalysts is crucial to the commercialization of rechargeable ZABs. Among various non-noble catalysts, metal-nitrogen-carbon (M-N-C) nanomaterials have attracted particular attention due to their low price, abundant reserves, excellent electrochemical activity and high stability," said Dr. Li.
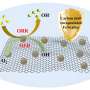
Creating an electrocatalyst composed of three different metal atoms isn't a trivial matter, however, due to the different interaction forces that occur with each metal atom. To address this issue, the team used zeolitic imidazolate frameworks (ZIFs), carbon-nitrogen frameworks that surround and arrange each of three metal atoms (Fe, Co and Ni), to uniformly anchor the catalytic atoms onto porous carbon at high heat.
The team confirmed the distribution of the Fe, Co and Ni atoms via energy-dispersive X-ray spectroscopy (EDX), spherical aberration-corrected high-angle annular dark-field scanning transmission electron microscopy (AC-HAADF-STEM) and electron energy loss spectroscopy (EELS).
Overall, the ternary Fe-Co-Ni electrocatalyst outperformed bimetal electrocatalysts (FeNi, FeCo and CoNi) and platinum and ruthenium, two noble metal electrocatalysts, in the oxygen reduction and evolution reactions. The team believes that all three metal atoms of the ternary electrocatalyst are active and cooperating to increase catalytic activity, with Fe contributing the most to activity as the most abundant atom. The porous structure and increased surface area of the electrocatalyst likely also contributes to the enhanced catalytic activity.
Overall, the team's rechargeable ZAB achieved a specific capacity of 846.8 mAh·gZn−1 and a impressive power density of 135 mW·cm–2 in liquid electrolyte. The ZAB also achieves a power density of 60 mW·cm–2 using the team's optimized CD/PVA solid-state electrolyte, which exceeds reported results of solid-state ZABs with other catalysts.
Importantly, the ZAB developed in the study was both durable and stable and capable of powering a fan and an LED screen and charging a mobile phone. The researchers are hopeful that their ternary Fe-Co-Ni electrocatalyst and CD/PVA electrolyte will spur investigations into new catalysts and electrolytes for practical, high-performance ZAB technologies.
Other contributors include Shifeng Qin, Mengxue Cao and Zhongyuan Huang from the College of Chemistry and Chemical Engineering at Hunan University in Changsha, China; Kaiqi Li, Guanjie He and Ivan P. Parkin from the Department of Chemistry at the University College London in London, UK; and Wuhua Liu from Guizhou Dalong Huicheng New Material Co., Ltd, in Tongren, China.
More information: Shifeng Qin et al, Fe-Co-Ni ternary single-atom electrocatalyst and stable quasi-solid-electrolyte enabling high-efficiency zinc-air batteries, Nano Research Energy (2024). DOI: 10.26599/NRE.2024.9120122
Provided by Tsinghua University Press