This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New approach sets stage to explore the mirror-image world of century-old naturally-occurring cyclodextrins
Cyclodextrins (CDs), a class of cyclic oligosaccharides that were "born" in 1891, have opened up endless research and commercial opportunities in numerous fields that span carbohydrate, supramolecular (host-guest) and analytical chemistry all the way from research laboratories in academia to the mass production of products—e.g., skin care enablers to drug delivery systems—in industry.
Although they have been known for over 130 years, the most accessible cyclic homologs are α-, β-, and γ-CDs, which contain six, seven, and eight D-glucopyranosyl units, respectively. The odyssey of naturally occurring CDs suggests that, despite the number of CDs being rather limited, their reach has been limitless.
For a long time, scientists have been exploring novel methods to synthesize—both chemically and enzymatically—CD homologues. These efforts include the syntheses of unusual smaller analogs with only 3, 4, and 5 D-glucose units and the making of rare larger CDs with 9 to 12 D-glucose units.
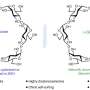
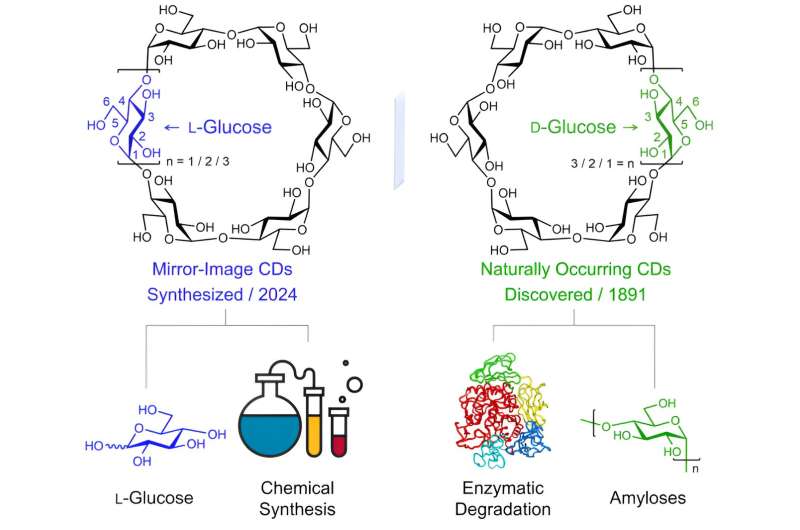
All the currently available CDs are composed solely of D-glucopyranosyl units as the monomers, while the syntheses of mirror-image CDs have remained an untouched goal of the CD community, limiting the realization of their full potential, such as in the development of new supramolecular sensors and catalysts, chiral materials, as well as innovative drug delivery systems and active pharmaceutical ingredients.
In order to fulfill this fundamental research niche, a collaborative research team led by Professor Sir Fraser STODDART in the Department of Chemistry of The University of Hong Kong (HKU) and Professor Daniel ARMSTRONG in the Department of Chemistry and Biochemistry of The University of Texas at Arlington, developed a concise approach to link L-glucopyranosyl monosaccharides together in a highly diastereoselective and scalable manner, resulting in the production of circa half-gram quantities of α-, β-, and γ-L-CDs.
The availability of L-CDs for the first time—ever since the serendipitous discovery of their natural counterparts back in 1891—has enabled the elucidation of an unprecedented chiral self-sorting of a racemic modification of β-CDs in the solid state and an investigation of the chiral recognition of enantiomeric guests by α-L-CD in water.
The research work was published and featured on the cover in the journal—Nature Synthesis. A corresponding News & Views Article written by Professor Sophie BEEREN, a cyclodextrin expert working at Technical University of Denmark, was also published in the same journal.
Innovative design
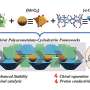
The team designed and synthesized two monosaccharide building blocks, both of which bear benzoyl groups as the source of protection of the primary hydroxyl groups, designated to direct the diastereoselective formation of 1,2-cis L-glucopyranosyl linkages through remote anchimeric assistance.
In order to simplify the assembly process of linear oligosaccharides, monosaccharide and disaccharide building blocks were allowed to react sequentially in a one-pot manner, resulting in the rapid construction of linear hexa-, hepta-, and octasaccharides involving only minimal intermediate isolation and protecting group manipulation.
The team carried out extensive optimization of conditions for the intramolecular glycosylations (cyclizations) which ended up with the high-yielding formation of fully protected α-, β-, and γ-L-CDs. Final global deprotection produced the three L-CDs in up to eight steps from simple and readily available monosaccharide building blocks.
"This piece of research, which showcases how synthetic carbohydrate chemistry can enrich the toolbox currently available for the CD community, constitutes a major milestone in the long history of CDs," commented Professor Sir Fraser Stoddart.
"One fundamental question to be answered is how the unnatural stereochemistry of L-CDs would affect those biomedically directed applications in which CDs are used as excipients in drug formulation and as active pharmaceutical ingredients?"
The team envisions that L-CDs should be more biologically stable than their natural counterparts on account of the fact that naturally occurring enzymes will not be able to recognize L-CDs.
Currently, the team is collaborating with biomedical scientists to evaluate systematically the biological effects of L-CDs using different cell lines and animal models as well as to explore potential applications in biological and biomedical research.
More information: Yong Wu et al, Mirror-image cyclodextrins, Nature Synthesis (2024). DOI: 10.1038/s44160-024-00495-8
Sophie R. Beeren, Synthesis of l-cyclodextrins, Nature Synthesis (2024). DOI: 10.1038/s44160-024-00512-w
Journal information: Nature Synthesis
Provided by The University of Hong Kong