April 16, 2024 report
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
L-cyclodextrins synthesized in the lab for the first time
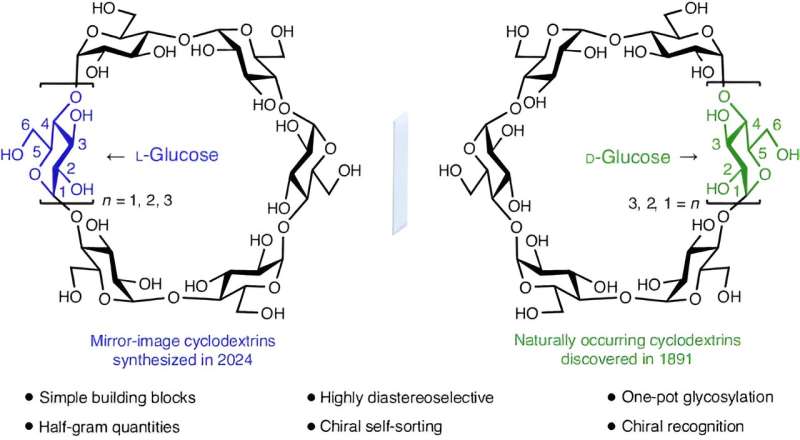
A team of chemists at Northwestern University has successfully synthesized three L-cyclodextrins in the lab for the first time. In their study, published in the journal Nature Synthesis, the group used a one-pot strategy to achieve the feat.
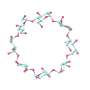
L-cyclodextrins are ringed sugar units that exist as mirror images of D-cyclodextrins, which are much more common. And D-cyclodextrins are part of a family of naturally occurring cyclic oligosaccharides, that have a macrocyclic ring of sugar-type subunits, all held together via glycosidic bonds.
They were first observed more than 130 years ago and are now typically produced from starches and have many applications, including pharmaceuticals and cosmetics. D-cyclodextrins can also be used to create luminescent materials that emit circularly polarized light, where electromagnetic waves rotate around the light vector, allowing for the creation of materials that can be used in applications such as chromatography. Prior to this new effort, very few efforts to synthesize mirror-image natural oligosaccharides have met with success.
Chemists have known for some time that L-cyclodextrins could be equally useful if they could be synthesized in the lab and produced commercially. In this new effort, the research team in Illinois has overcome the first part of the problem—synthesizing them in a way that is both reproducible and scalable.
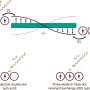
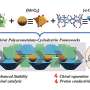
The work involved a one-pot glycosylation strategy, one that involved creating linear oligosaccharides with specific protecting groups followed by sequential glycosylation. The team used p-PhMeSCl and AgOTf as promoters (which they describe as the key to achieving high diastereoselectivity), along with diethylether as a solvent. The result was significant quantities of six- or eight-ring α-l-, β-l-, and γ-l-cyclodextrins. Deprotection of the results, the team reports, produced yields higher than 75%.
The team says the eight-step method they used allows for efficient and scalable synthesis of three types of L-cyclodextrins in a straightforward and easily reproducible manner. They also note that the L-cyclodextrins they produced thus far appear to be more stable than D-cyclodextrins.
More information: Yong Wu et al, Mirror-image cyclodextrins, Nature Synthesis (2024). DOI: 10.1038/s44160-024-00495-8
Journal information: Nature Synthesis
© 2024 Science X Network