This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
Research team makes hydroxylamine from air and water
A research team led by Prof. Zeng Jie and Prof Geng Zhigang from University of Science and Technology of China (USTC) has proposed a new model for sustainable hydroxylamine (NH2OH) synthesis via a plasma-electrochemical cascade pathway (PECP). They achieved the green and sustainable synthesis of NH2OH from ambient air and water at mild conditions. Their study is published in Nature Sustainability.
NH2OH, as an important chemical intermediate, is widely used in the fine chemical fields of medicine, pesticides, textiles, and more. The traditional production methods of NH2OH mainly include the Raschig process, a nitric oxide reduction method, and a nitric acid reduction method. However, the Raschig process causes a large amount of nitrogen loss and environmental pollution; while the other two methods give out great carbon emissions. It is therefore urgent to develop a new green, low-carbon and sustainable synthesis process for NH2OH.
The electrosynthesis process driven by green electricity and using water as a proton source is expected to overcome the drawbacks of traditional NH2OH production processes. However, due to the thermodynamic stability of nitrogen molecules, it is difficult to achieve efficient activation of nitrogen molecules in the direct electrocatalytic process of nitrogen.
The researchers have achieved the green and sustainable synthesis of NH2OH using only air and water as raw materials by developing a new process that couples plasma nitrogen fixation to nitric acid production with the electrocatalytic reduction of nitric acid to NH2OH. In addition, the team has designed a plasma discharge device with multiple parallel tips so as to enlarge the overlapping zone for the efficient activation of nitrogen gas.
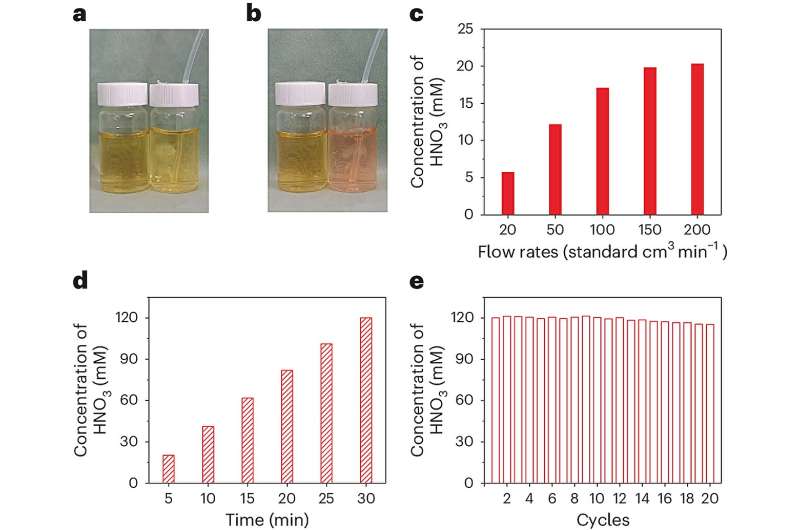
First, the researchers introduced air into the plasma parallel arc discharge device and used a water solution containing methyl orange as the exhaust gas absorbent to convert the solution from neutral to acidic. By optimizing the air flow rate, they obtained a nitric acid solution with a maximum concentration of 20.3 millimoles per liter. With each reaction cycle lasting 30 minutes, the plasma discharge device maintained excellent stability over 20 cycles. The obtained nitric acid solution could be directly used for the electrocatalytic synthesis of NH2OH after dilution and addition of electrolytes.
In addition, the team also prepared a bismuth metal thin film catalyst via magnetron sputtering and applied it to the electrocatalytic reduction of nitric acid to produce NH2OH.
The accumulation of NH2OH in the electrolyte during long-term electrolysis of a 100 mmol/L nitric acid solution by a bismuth thin film catalyst was investigated. After continuous electrolysis for 5 h, the highest concentration of NH2OH reached 77.7 mmol/L. Finally, 1.887 g high-purity NH2OH sulfate product was prepared.
This study proposes a viable way to efficiently synthesize hydroxylamine from simpler feedstock at milder conditions, which contributes to the sustainability transformation of the chemical industry.
More information: Xiangdong Kong et al, Synthesis of hydroxylamine from air and water via a plasma-electrochemical cascade pathway, Nature Sustainability (2024). DOI: 10.1038/s41893-024-01330-w
Journal information: Nature Sustainability
Provided by University of Science and Technology of China