This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers reveal how acetylation regulates centromere dynamics, chromosome segregation and mitotic progression
Cell division produces new cells, which underpin life proliferation and development. Mitosis is the shortest and the most dynamic phase of the cell cycle. During mitosis, chromosomes are evenly distributed between the two daughter cells, preserving the integrity of the genome.
The centromere, a specialized region of the chromosome, serves as a recruitment platform for diverse proteins crucial for maintaining sister chromatid cohesion and for the assembly of kinetochores. These kinetochores are responsible for the attachment of spindle microtubules, which enable chromosome congression and proper segregation during cell division.
Defects in the structure or function of the centromere can lead to chromosome missegregation, which may in turn result in genomic instability.
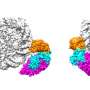
Bub1 (Budding Uninhibited by Benzimidazole 1) is a serine/threonine kinase that performs diverse functions during mitosis. It is recruited to unattached kinetochores during prometaphase and promotes the recruitment of other downstream proteins to the kinetochores and centromeres.
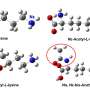
Protein acetylation is a conserved post-translational modification that exists in both prokaryotes and eukaryotes. In 1964, researchers first discovered the acetylation of histones. Subsequent studies revealed that not only histones but also numerous non-histone proteins are subject to acetylation modifications, which are involved in the regulation of various cellular life activities.
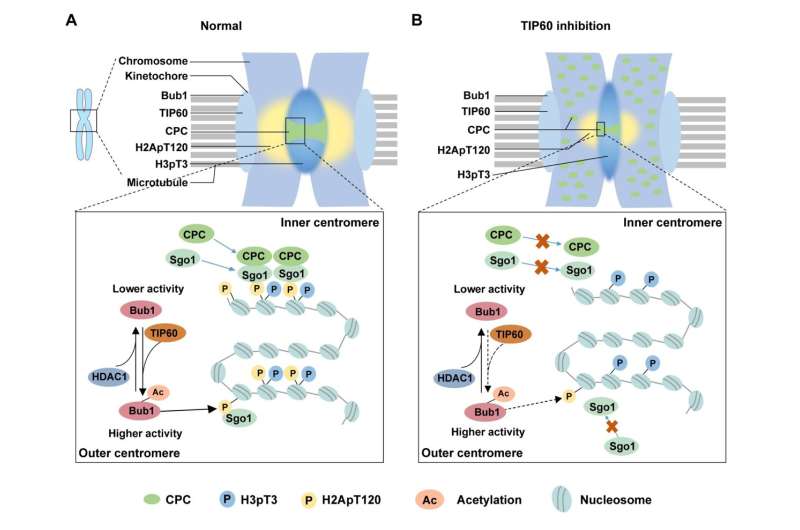
Prof. Chuanmao Zhang and colleagues, at Kunming University of Science and Technology and Peking University, have found a molecular mechanism that regulates chromosome congression and segregation. The researchers have shown in their paper published in Science China Life Sciences, TIP60 acetylates Bub1 at K424 and K431 on kinetochores during early mitosis, which enhances the kinase activity of Bub1.
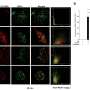
"When we enriched the mitotic acetylated proteins, we detected Bub1 among them," said Mengjie Sun, the first author of the paper. "Furthermore, we observed a significant surge in the acetylation level of Bub1 during early mitosis. This suggests that Bub1 acetylation might have a role to play in the process."
This is the beginning of their research. And then, through a series of experiments, which include live cell imaging and biochemical techniques, they discovered that Bub1 acetylation is essential for proper chromosome congression and segregation. Impaired Bub1 acetylation causes a significant delay in chromosome alignment and defects in chromosome segregation, including lagging chromosomes and anaphase bridge formation.
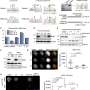
Next, through in vivo and in vitro experiments, they determined that acetyltransferase TIP60 is responsible for the Bub1 acetylation.
Furthermore, the researchers delved into investigating how Bub1 acetylation, mediated by TIP60, regulates chromosome congression and segregation. They detected the impact on the recruitment of downstream proteins to the centromeres and found that the CPC components and Sgo1 were unable to be effectively recruited to the centromeres when Bub1 acetylation was disrupted.
Previous studies have reported that Bub1 phosphorylates H2A at T120 (H2ApT120) to recruit CPC and Sgo1. The researchers then sought to determine how Bub1 acetylation affects the level of H2ApT120. As they expected, Bub1 acetylation enhances its kinase activity, thereby promoting the phosphorylation of H2A.
"This is the mechanism by which Bub1 acetylation regulates chromosome congression and segregation," Prof. Zhang concluded.
Given that the acetylation of Bub1 is a dynamic process throughout the cell cycle, they also aimed to identify the enzyme responsible for deacetylating Bub1. They pinpointed an essential deacetylase, HDAC1, which mediates the deacetylation of Bub1 when cells exit mitosis. Premature deacetylation of Bub1 by HDAC1 can impair the kinase activity of Bub1 and lead to abnormal centromere disassembly and chromosome missegregation.
"This forms an acetylation and deacetylation cycle of Bub1 in the cell cycle," Dr. Biying Yang explained.
More information: Mengjie Sun et al, TIP60 acetylation of Bub1 regulates centromeric H2AT120 phosphorylation for faithful chromosome segregation, Science China Life Sciences (2024). DOI: 10.1007/s11427-023-2604-8
Journal information: Science China Life Sciences
Provided by Science China Press