This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
The science of static shock jolted into the 21st century
Shuffling across the carpet to zap a friend may be the oldest trick in the book, but on a deep level that prank still mystifies scientists, even after thousands of years of study.
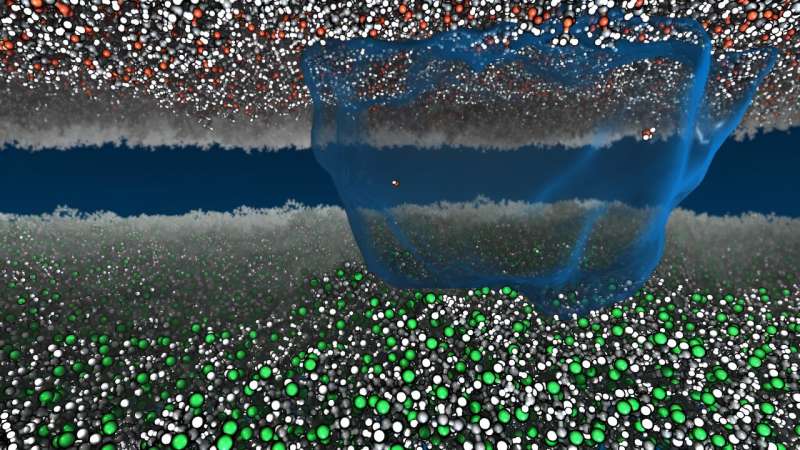
Now Princeton researchers have sparked new life into static. Using millions of hours of computational time to run detailed simulations, the researchers found a way to describe static charge atom-by-atom with the mathematics of heat and work. Their paper, "Thermodynamic driving forces in contact electrification between polymeric materials," appears in Nature Communications.
The study looked specifically at how charge moves between materials that do not allow the free flow of electrons, called insulating materials, such as vinyl and acrylic. The researchers said there is no established view on what mechanisms drive these jolts, despite the ubiquity of static: the crackle and pop of clothes pulled from a dryer, packing peanuts that cling to a box.
"We know it's not electrons," said Mike Webb, assistant professor of chemical and biological engineering, who led the study. "What is it?"
Webb first asked himself that question as a postdoctoral researcher at the University of Chicago. He puzzled over it with colleagues, baffled that such a common phenomenon could be so poorly understood. But the more they looked, the more insurmountable the questions became. "It just seemed out of reach," he said.
It had been out of reach since Thales of Miletus first rubbed amber with fur and watched the amber (Greek: elektron) collect feathers and dust—26 centuries ago. Thales was one of the first people to explain nature through reason rather than supernatural forces. He played a critical role in the development of philosophy and eventually science. Despite the depth and breadth of knowledge accumulated over subsequent millennia, despite the myriad technologies born of that knowledge, science, in all that time, never cracked static. Maybe it never would.
At Princeton, Webb got to talking to his colleague Sankaran Sundaresan, a leading expert in chemical reaction engineering who specializes in the flow of materials in gaseous chambers. In those environments, loaded with volatile chemicals, a stray spark could be deadly. Sundaresan had worked with static charge for decades, using reliable experimental data to predict but not fully fathom how charge moved in these systems.
"I treat that like a black box," said Sundaresan, the Norman John Sollenberger Professor in Engineering. "We do some experiments and the experiments tell me: This is what happens. This is the charge." He works down to the limit and carefully notes what he sees. What happens inside the black box remains a mystery.
One thing you find no matter where you look, though, according to Sundaresan, is trace amounts of water. Charged water molecules are everywhere, in nearly everything, clinging to virtually every surface on Earth. Even in extremely arid conditions, under intense heat, stray water ions pool into microscopic oases that harbor electrical charge.
Incidentally, Thales is best known not for his work on electricity but for an even grander project. He proposed that the entirety of nature was made of water, that water was the Ur-substance, the essential stuff. It was the first attempt at a unified theory of everything. Aristotle wrote it all down.
Over the arc of Sundaresan's career, he and his colleagues shrunk that black box so that the mysteries have been pushed ever deeper. But mysteries they remain.
The conversation between he and Webb led to a mutual realization. Sundaresan had decades of insight into data from reactors, and Webb could apply sophisticated atom-scale computational techniques to look at these water ions from the perspective of thermodynamics.
How much energy would it take for a water ion to bolt from surface to surface? Maybe that would explain what was happening inside Sundaresan's black box. The unresolved puzzle from Webb's postdoc days came unlocked.
By modeling the relationship between charged water molecules and the amount of energy those molecules have available to propel them between surfaces, Webb and graduate student Hang Zhang demonstrated a very precise mathematical approximation of how electrical charge moves between two insulating materials.
In other words, they used math to simulate the movement of around 80,000 atoms. Those simulations matched real-life observations with a very high degree of precision. It turns out, in all likelihood, static shock is a function of water, and more specifically, the free energy of stray water ions.
With that framework, Webb and Zhang revealed the molecular underpinnings of those familiar shocks in infinitesimal detail. They blew Sundaresan's black box wide open. If only Thales could see.
More information: Hang Zhang et al, Thermodynamic driving forces in contact electrification between polymeric materials, Nature Communications (2024). DOI: 10.1038/s41467-024-46932-2
Journal information: Nature Communications
Provided by Princeton University