This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Palladium nanocluster catalyst supports highly efficient and regioselective hydrogenation of epoxides
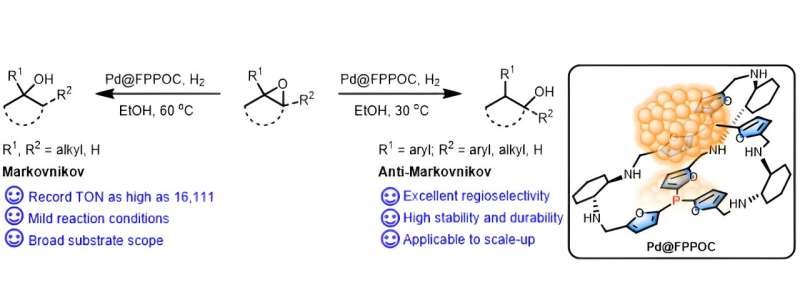
Alcohols are widely applied in life sciences and the chemical industry. Selective hydrogenation of epoxides using hydrogen molecules as a reductant is considered to be one of the most facile and atom-economical strategies for alcohol synthesis. However, controlling the regioselective ring opening of epoxides remains a challenge.
Significant progress has been made in the selective hydrogenation of epoxides using homogeneous catalysis. However, challenges remain in the difficult separation and recovery of the catalyst, as well as the drawbacks of requiring expensive and sophisticated ligands, which severely limit their practical potential. Therefore, the development of efficient and highly regioselective heterogeneous catalysts for epoxide hydrogenation is particularly important.
A palladium (Pd) nanocluster catalyst for the selective hydrogenation of epoxides has been developed by Yang Yong from the Qingdao Institute of Bioenergy and Bioprocess Technology of the Chinese Academy of Sciences.
The results were published in CCS Chemistry.
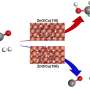
The modulation of electronic effects and spatial structure of phosphine ligands led to the design and synthesis of a novel porous organic cage (FPPOC). This cage was used as both ligand and to support the preparation of a Pd nanocluster catalyst named Pd@FPPOC.
The results indicate that the interaction between organic phosphine and Pd nanoclusters (Pd NCs) has resulted in the uniform dispersion of ultrafine Pd NCs on FPPOC. This interaction effectively stabilizes the Pd NCs, prevents their oxidation and aggregation, and significantly increases the surface electron density of Pd NCs, thereby enhancing the catalytic performance.
By systematically optimizing the conditions, the efficient conversion of aromatic epoxides to linear alcohols and aliphatic epoxides to branched alcohols was achieved using Pd@FPPOC. The success can be attributed to the robust coordination effect of phosphorus within the molecular cage, coupled with the advantageous geometric structure of the porous organic cage.
Various terminal and internal epoxides can be efficiently hydrogenated to the corresponding linear or branched alcohols with excellent functional group tolerance. The catalyst exhibits remarkably high catalytic activity (TON > 16,000) and stability (retains activity and selectivity after 10 cycles of use), and it facilitates the scalable synthesis of phenylethanol at the 100 mmol scale.
The mechanism of hydrogenation of aromatic and aliphatic epoxides by Pd@FPPOC was elucidated by control experiments and density functional theory calculations.
More information: Xin Zhou et al, Phosphine-Built-In Porous Organic Cage Supported Ultrafine Pd Nanoclusters Enable Highly Efficient and Regioselective Hydrogenation of Epoxides, CCS Chemistry (2024). DOI: 10.31635/ccschem.024.202303468
Provided by Chinese Academy of Sciences