This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Researchers develop better way to make painkiller from trees

Scientists at the University of Wisconsin–Madison have developed a cost-effective and environmentally sustainable way to make a popular pain reliever and other valuable products from plants instead of petroleum.
Building on a previously patented method for producing paracetamol—the active ingredient in Tylenol—the discovery promises a greener path to one of the world's most widely used medicines and other chemicals. More importantly, it could provide new revenue streams to make cellulosic biofuels—derived from non-food plant fibers—cost competitive with fossil fuels, the primary driver of climate change.
"We did the R&D to scale it and make it realizable," says Steven Karlen, a staff scientist at the Great Lakes Bioenergy Research Center who led the research published recently in the journal ChemSusChem.
Paracetamol, also known as acetaminophen, is one of the most widely used pharmaceuticals, with a global market value of about $130 million a year. Since it was introduced in the early 1900s, the drug has traditionally been made from derivatives of coal tar or petroleum.
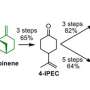
In 2019, Karlen and UW–Madison biochemistry professor John Ralph showed how it could be made instead from a compound in poplar trees using a well-known chemical reaction.
Now Karlen's team has improved the process for making paracetamol as well as other drugs, pigments, textiles, and biodegradable plastics with a cumulative market value of more than $1.5 billion, a portfolio of products that Karlen says could support dozens of small biorefineries feeding into larger hubs without saturating the market.
The process is available for commercial licensing through the Wisconsin Alumni Research Foundation, the nonprofit organization that commercializes university discoveries to support ongoing research.
The paracetamol molecule is made of a six-carbon benzene ring with two chemical groups attached. Poplar trees produce a similar compound called p-hydroxybenzoate (pHB) in lignin, the part of the cell wall that binds plant sugars together and provides structure.
Lignin is chock full of valuable aromatic compounds that could replace many petrochemicals and provide biorefineries with additional revenue streams to make plant-based fuels cost-competitive. The challenge is breaking down the complex and irregular chain of molecules into useful components.
It turns out that pHB is relatively easy to break off with chemical treatment, but while the initial discovery showed it was chemically possible to turn it into paracetamol, Karlen says that the process didn't convert enough of the raw material into the final product.
Research scientist Vitaliy Tymokhin discovered that treating poplar biomass with a different—and typically cheaper—method converted nearly all the pHB into another chemical that can then be converted into paracetamol or a less valuable molecule with other applications.
"You can make dyes like black ink, polymers which can be used in textiles or material application, convert it to adhesives or into stuff like that," Karlen says. "It's got a huge market and big value."
By recycling the unreacted product back through in a continuous reactor, the scientists successfully converted 90% of the raw material into paracetamol, which they extracted using a method that's cheaper than traditional purification techniques. Karlen says it should be possible to dial the yield up to 99%.
The process is primarily water-based, relies on green solvents, and is continuous rather than a batch reaction, which makes it ideal for industrial applications.
"As I'm chopping the tree up, it can feed right into a reactor that pulls out the benzamide," Karlen says. "So you're never stopping. As fast as your trucks can come in and fill that hopper, you can keep processing."
More information: Steven D Karlen et al, Production of biomass‐derived p‐hydroxybenzamide: Synthesis of p‐aminophenol and paracetamol, ChemSusChem (2024). DOI: 10.1002/cssc.202400234
Provided by University of Wisconsin-Madison